The HRA2pl fusion peptide exerts in vitro antiviral activity against human respiratory paramyxoviruses and pneumoviruses
- PMID: 37153148
- PMCID: PMC10157160
- DOI: 10.3389/fcimb.2023.1125135
The HRA2pl fusion peptide exerts in vitro antiviral activity against human respiratory paramyxoviruses and pneumoviruses
Abstract
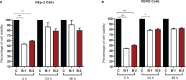
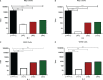
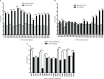
Acute respiratory infections are a group of diseases caused by viruses, bacteria, and parasites that mainly affect children until the age of 5 and immunocompromised senior adults. In Mexico, these infections are the main cause of morbidity in children, with more than 26 million cases of respiratory infections reported by the Secretariat of Health, in 2019. The human respiratory syncytial virus (hRSV), the human metapneumovirus (hMPV), and the human parainfluenza-2 (hPIV-2) are responsible for many respiratory infections. Currently, palivizumab, a monoclonal antibody against the fusion protein F, is the treatment of choice against hRSV infections. This protein is being studied for the design of antiviral peptides that act by inhibiting the fusion of the virus and the host cell. Therefore, we examined the antiviral activity of the HRA2pl peptide, which competes the heptad repeat A domain of the F protein of hMPV. The recombinant peptide was obtained using a viral transient expression system. The effect of the fusion peptide was evaluated with an in vitro entry assay. Moreover, the effectiveness of HRA2pl was examined in viral isolates from clinical samples obtained from patients with infections caused by hRSV, hMPV, or hPIV-2, by evaluating the viral titer and the syncytium size. The HRA2pl peptide affected the viruses' capacity of entry, resulting in a 4-log decrease in the viral titer compared to the untreated viral strains. Additionally, a 50% reduction in the size of the syncytium was found. These results demonstrate the antiviral potential of HRA2pl in clinical samples, paving the way toward clinical trials.
Keywords: acute respiratory infections; fusion peptide; human respiratory virus; paramyxovirus; pneumovirus; syncytium size.
Copyright © 2023 Meza, Lara, Gómez, Rodríguez, Hernández and Mendoza.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Cerezo L. G., Zárate C. K., Alpuche-Lazcano S., et al. (2016). Diagnóstico molecular para la detección de metapneumovirus humano a partir de aislados virales en pacientes pediátricos con infección respiratoria aguda. Investigación en Discapacidad 5 (2), 88–95.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

