Role of macrophages in pulmonary arterial hypertension
- PMID: 37153557
- PMCID: PMC10154553
- DOI: 10.3389/fimmu.2023.1152881
Role of macrophages in pulmonary arterial hypertension
Abstract
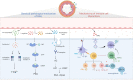
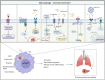
Pulmonary arterial hypertension (PAH) is a severe cardiopulmonary vascular disease characterized by progressive pulmonary artery pressure elevation, increased pulmonary vascular resistance and ultimately right heart failure. Studies have demonstrated the involvement of multiple immune cells in the development of PAH in patients with PAH and in experimental PAH. Among them, macrophages, as the predominant inflammatory cells infiltrating around PAH lesions, play a crucial role in exacerbating pulmonary vascular remodeling in PAH. Macrophages are generally polarized into (classic) M1 and (alternative) M2 phenotypes, they accelerate the process of PAH by secreting various chemokines and growth factors (CX3CR1, PDGF). In this review we summarize the mechanisms of immune cell action in PAH, as well as the key factors that regulate the polarization of macrophages in different directions and their functional changes after polarization. We also summarize the effects of different microenvironments on macrophages in PAH. The insight into the interactions between macrophages and other cells, chemokines and growth factors may provide important clues for the development of new, safe and effective immune-targeted therapies for PAH.
Keywords: Pulmonary arterial hypertension; inflammatory response; macrophage microenvironment; macrophages; vascular remodeling.
Copyright © 2023 Zhang, Wang, Pang, Shi, Li, Xie, Wang, Zhang, Zhou, Chen, Han, Zhao and He.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

