Immunomodulatory effects of bacteriocinogenic and non-bacteriocinogenic Lactococcus cremoris of aquatic origin on rainbow trout (Oncorhynchus mykiss, Walbaum)
- PMID: 37153602
- PMCID: PMC10159052
- DOI: 10.3389/fimmu.2023.1178462
Immunomodulatory effects of bacteriocinogenic and non-bacteriocinogenic Lactococcus cremoris of aquatic origin on rainbow trout (Oncorhynchus mykiss, Walbaum)
Abstract
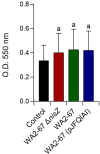
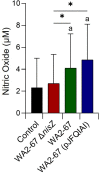
Lactic Acid Bacteria (LAB) are a group of bacteria frequently proposed as probiotics in aquaculture, as their administration has shown to confer positive effects on the growth, survival rate to pathogens and immunological status of the fish. In this respect, the production of antimicrobial peptides (referred to as bacteriocins) by LAB is a common trait thoroughly documented, being regarded as a key probiotic antimicrobial strategy. Although some studies have pointed to the direct immunomodulatory effects of these bacteriocins in mammals, this has been largely unexplored in fish. To this aim, in the current study, we have investigated the immunomodulatory effects of bacteriocins, by comparing the effects of a wild type nisin Z-expressing Lactococcus cremoris strain of aquatic origin to those exerted by a non-bacteriocinogenic isogenic mutant and a recombinant nisin Z, garvicin A and Q-producer multi-bacteriocinogenic strain. The transcriptional response elicited by the different strains in the rainbow trout intestinal epithelial cell line (RTgutGC) and in splenic leukocytes showed significant differences. Yet the adherence capacity to RTgutGC was similar for all strains. In splenocyte cultures, we also determined the effects of the different strains on the proliferation and survival of IgM+ B cells. Finally, while the different LAB elicited respiratory burst activity similarly, the bacteriocinogenic strains showed an increased ability to induce the production of nitric oxide (NO). The results obtained reveal a superior capacity of the bacteriocinogenic strains to modulate different immune functions, pointing to a direct immunomodulatory role of the bacteriocins, mainly nisin Z.
Keywords: bacteriocins; fish; immunomodulation; lactic acid bacteria; nisin; probiotics.
Copyright © 2023 Contente, Díaz-Rosales, Feito, Díaz-Formoso, Docando, Simón, Borrero, Hernández, Poeta, Muñoz-Atienza, Cintas and Tafalla.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Nisin Z Production by Lactococcus lactis subsp. cremoris WA2-67 of Aquatic Origin as a Defense Mechanism to Protect Rainbow Trout (Oncorhynchus mykiss, Walbaum) Against Lactococcus garvieae.Mar Biotechnol (NY). 2015 Dec;17(6):820-30. doi: 10.1007/s10126-015-9660-x. Epub 2015 Aug 26. Mar Biotechnol (NY). 2015. PMID: 26307018
-
Design of Lactococcus lactis Strains Producing Garvicin A and/or Garvicin Q, Either Alone or Together with Nisin A or Nisin Z and High Antimicrobial Activity against Lactococcus garvieae.Foods. 2023 Mar 2;12(5):1063. doi: 10.3390/foods12051063. Foods. 2023. PMID: 36900581 Free PMC article.
-
Draft Genome Sequence of Lactococcus lactis Subsp. cremoris WA2-67: A Promising Nisin-Producing Probiotic Strain Isolated from the Rearing Environment of a Spanish Rainbow Trout (Oncorhynchus mykiss, Walbaum) Farm.Microorganisms. 2022 Feb 28;10(3):521. doi: 10.3390/microorganisms10030521. Microorganisms. 2022. PMID: 35336097 Free PMC article.
-
Nisin-A lantibiotic with immunomodulatory properties: A review.Peptides. 2021 Mar;137:170479. doi: 10.1016/j.peptides.2020.170479. Epub 2020 Dec 30. Peptides. 2021. PMID: 33359393 Review.
-
Microbial production of bacteriocins: Latest research development and applications.Biotechnol Adv. 2018 Dec;36(8):2187-2200. doi: 10.1016/j.biotechadv.2018.10.007. Epub 2018 Oct 29. Biotechnol Adv. 2018. PMID: 30385277 Review.
Cited by
-
Antimicrobial Activity, Genetic Relatedness, and Safety Assessment of Potential Probiotic Lactic Acid Bacteria Isolated from a Rearing Tank of Rotifers (Brachionus plicatilis) Used as Live Feed in Fish Larviculture.Animals (Basel). 2024 May 9;14(10):1415. doi: 10.3390/ani14101415. Animals (Basel). 2024. PMID: 38791633 Free PMC article.
-
Effects of Dietary Fish Oil Levels on Growth Performance, Lipid Metabolism, Hepatic Health, Nonspecific Immune Response, and Intestinal Microbial Community of Juvenile Amur Grayling (Thymallus grubii).Aquac Nutr. 2024 Nov 21;2024:8587410. doi: 10.1155/anu/8587410. eCollection 2024. Aquac Nutr. 2024. PMID: 39619108 Free PMC article.
-
Terminalia arjuna Bark Powder as a Potential Immunomodulator in Labeo rohita: Enhanced Hematological, Adaptive, and Humoral Responses against Bacterial Pathogens and Concordant Liver Histomorphology.Pathogens. 2024 Mar 30;13(4):295. doi: 10.3390/pathogens13040295. Pathogens. 2024. PMID: 38668250 Free PMC article.
-
Genomic Sequence of Streptococcus salivarius MDI13 and Latilactobacillus sakei MEI5: Two Promising Probiotic Strains Isolated from European Hakes (Merluccius merluccius, L.).Vet Sci. 2024 Aug 10;11(8):365. doi: 10.3390/vetsci11080365. Vet Sci. 2024. PMID: 39195819 Free PMC article.
-
Genomic and Functional Evaluation of Two Lacticaseibacillus paracasei and Two Lactiplantibacillus plantarum Strains, Isolated from a Rearing Tank of Rotifers (Brachionus plicatilis), as Probiotics for Aquaculture.Genes (Basel). 2024 Jan 1;15(1):64. doi: 10.3390/genes15010064. Genes (Basel). 2024. PMID: 38254954 Free PMC article.
References
-
- Food and Agriculture Organization (FAO) . The state of the world fisheries and aquaculture. In: Towards blue transformation. Rome, Italy: FAO; (2022). doi: 10.4060/cc0461en - DOI
-
- Kiron V. Fish immune system and its nutritional modulation for preventive health care. Anim Feed Sci Technol (2012) 173:111–33. doi: 10.1016/j.anifeedsci.2011.12.015 - DOI
-
- Lulijwa R, Rupia EJ, Alfaro AC. Antibiotic use in aquaculture, policies and regulation, health and environmental risks: a review of the top 15 major producers. Rev Aquac (2020) 12:640–63. doi: 10.1111/raq.12344 - DOI
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Miscellaneous

