A strategy for high antibody expression with low anti-drug antibodies using AAV9 vectors
- PMID: 37153616
- PMCID: PMC10161250
- DOI: 10.3389/fimmu.2023.1105617
A strategy for high antibody expression with low anti-drug antibodies using AAV9 vectors
Abstract
Introduction: Use of adeno-associated virus (AAV) vectors is complicated by host immune responses that can limit transgene expression. Recent clinical trials using AAV vectors to deliver HIV broadly neutralizing antibodies (bNAbs) by intramuscular administration resulted in poor expression with anti-drug antibodies (ADA) responses against the bNAb.
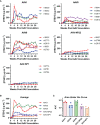
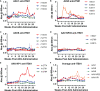
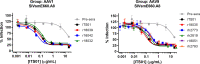
Methods: Here we compared the expression of, and ADA responses against, an anti-SIV antibody ITS01 when delivered by five different AAV capsids. We first evaluated ITS01 expression from AAV vectors three different 2A peptides. Rhesus macaques were selected for the study based on preexisiting neutralizing antibodies by evaluating serum samples in a neutralization assay against the five capsids used in the study. Macaques were intramuscularly administered AAV vectors at a 2.5x10^12 vg/kg over eight administration sites. ITS01 concentrations and anti-drug antibodies (ADA) were measured by ELISA and a neutralization assay was conducted to confirm ex vivo antibody potency.
Results: We observed that ITS01 expressed three-fold more efficiently in mice from AAV vectors in which heavy and light-chain genes were separated by a P2A ribosomal skipping peptide, compared with those bearing F2A or T2A peptides. We then measured the preexisting neutralizing antibody responses against three traditional AAV capsids in 360 rhesus macaques and observed that 8%, 16%, and 42% were seronegative for AAV1, AAV8, and AAV9, respectively. Finally, we compared ITS01 expression in seronegative macaques intramuscularly transduced with AAV1, AAV8, or AAV9, or with the synthetic capsids AAV-NP22 or AAV-KP1. We observed at 30 weeks after administration that AAV9- and AAV1-delivered vectors expressed the highest concentrations of ITS01 (224 µg/mL, n=5, and 216 µg/mL, n=3, respectively). The remaining groups expressed an average of 35-73 µg/mL. Notably, ADA responses against ITS01 were observed in six of the 19 animals. Lastly, we demonstrated that the expressed ITS01 retained its neutralizing activity with nearly the same potency of purified recombinant protein.
Discussion: Overall, these data suggest that the AAV9 capsid is a suitable choice for intramuscular expression of antibodies in nonhuman primates.
Keywords: AAV (adeno-associated virus); HIV - human immunodeficiency virus; SIV - simian immunodeficiency virus; anti-drug antibodies (ADA); antibody.
Copyright © 2023 Davis-Gardner, Weber, Xie, Pekrun, Alexander, Weisgrau, Furlott, Rakasz, Kay, Gao, Farzan and Gardner.
Conflict of interest statement
MG and MF are co-founders and consultants for Emmune, Inc. MG and MF are inventors on patents with potential royalties licensed to Emmune, Inc. KP and MK are named on patent applications for AAV variants used in this paper. MK has equity interests in LogicBio Therapeutics. GG is a co-founder of Voyager Therapeutics and Aspa Therapeutics and holds equity in both companies. GG is an inventor on patents with potential royalties licensed to Voyager Therapeutics, Aspa Therapeutics, and other biopharmaceutical companies. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Saunders KO, Wang L, Joyce MG, Yang Z-Y, Balazs AB, Cheng C, et al. Broadly neutralizing human immunodeficiency virus type 1 antibody gene transfer protects nonhuman primates from mucosal simian-human immunodeficiency virus infection. J Viro (2015) 89(16):8334–45. doi: 10.1128/JVI.00908-15 - DOI - PMC - PubMed
-
- Fuchs SP, Martinez-Navio JM, Piatak M, Jr., Lifson JD, Gao G, Desrosiers RC. AAV-delivered antibody mediates significant protective effects against SIVmac239 challenge in the absence of neutralizing activity. PloS Pathogens (2015) 11(8):e1005090. doi: 10.1371/journal.ppat.1005090 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

