A novel NEDD4L-TXNIP-CHOP axis in the pathogenesis of nonalcoholic steatohepatitis
- PMID: 37153733
- PMCID: PMC10157740
- DOI: 10.7150/thno.81192
A novel NEDD4L-TXNIP-CHOP axis in the pathogenesis of nonalcoholic steatohepatitis
Abstract
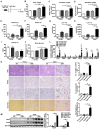
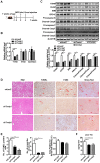
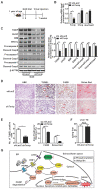
Background: Nonalcoholic steatohepatitis (NASH) is a leading cause of chronic liver diseases worldwide. There is a pressing clinical need to identify potential therapeutic targets for NASH treatment. Thioredoxin interacting protein (Txnip) is a stress responsive gene that has been implicated in the pathogenesis of NASH, but its exact role is not fully understood. Here, we investigated the liver- and gene-specific role of Txnip and its upstream/downstream signaling in the pathogenesis of NASH. Methods and Results: Using four independent NASH mouse models, we found that TXNIP protein abnormally accumulated in NASH mouse livers. A decrease in E3 ubiquitin ligase NEDD4L resulted in impaired TXNIP ubiquitination and its accumulation in the liver. TXNIP protein levels were positively correlated with that of CHOP, a major regulator of ER stress-mediated apoptosis, in NASH mouse liver. Moreover, gain- and loss-of-function studies showed that TXNIP increased protein not mRNA levels of Chop both in vitro and in vivo. Mechanistically, the C-terminus of TXNIP associated with the N-terminus of the α-helix domain of CHOP and decreased CHOP ubiquitination, thus increasing the stability of CHOP protein. Lastly, selective knockdown of Txnip by adenovirus-mediated shRNA (not targets Txnip antisense lncRNA) delivery in the livers of both young and aged NASH mice suppressed the expression of CHOP and its downstream apoptotic pathway, and ameliorated NASH by reducing hepatic apoptosis, inflammation, and fibrosis. Conclusions: Our study revealed a pathogenic role of hepatic TXNIP in NASH and identified a novel NEDD4L-TXNIP-CHOP axis in the pathogenesis of NASH.
Keywords: CHOP; E3 ligase; NASH; TXNIP; Ubiquitination.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





References
-
- Greenfield V, Cheung O, Sanyal AJ. Recent advances in nonalcholic fatty liver disease. Curr Opin Gastroenterol. 2008;24:320–7. - PubMed
-
- Wree A, Broderick L, Canbay A, Hoffman HM, Feldstein AE. From NAFLD to NASH to cirrhosis-new insights into disease mechanisms. Nat Rev Gastroenterol Hepatol. 2013;10:627–36. - PubMed
-
- Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59:2188–95. - PubMed
-
- Cusi K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology. 2012;142:711–25. e6. - PubMed
-
- Tiniakos DG, Vos MB, Brunt EM. Nonalcoholic fatty liver disease: pathology and pathogenesis. Annu Rev Pathol. 2010;5:145–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

