Role of voltage-gated proton channel (Hv1) in cancer biology
- PMID: 37153807
- PMCID: PMC10157179
- DOI: 10.3389/fphar.2023.1175702
Role of voltage-gated proton channel (Hv1) in cancer biology
Abstract
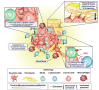
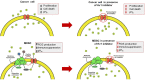
The acid-base characteristics of tumor cells and the other elements that compose the tumor microenvironment have been topics of scientific interest in oncological research. There is much evidence confirming that pH conditions are maintained by changes in the patterns of expression of certain proton transporters. In the past decade, the voltage-gated proton channel (Hv1) has been added to this list and is increasingly being recognized as a target with onco-therapeutic potential. The Hv1 channel is key to proton extrusion for maintaining a balanced cytosolic pH. This protein-channel is expressed in a myriad of tissues and cell lineages whose functions vary from producing bioluminescence in dinoflagellates to alkalizing spermatozoa cytoplasm for reproduction, and regulating the respiratory burst for immune system response. It is no wonder that in acidic environments such as the tumor microenvironment, an exacerbated expression and function of this channel has been reported. Indeed, multiple studies have revealed a strong relationship between pH balance, cancer development, and the overexpression of the Hv1 channel, being proposed as a marker for malignancy in cancer. In this review, we present data that supports the idea that the Hv1 channel plays a significant role in cancer by maintaining pH conditions that favor the development of malignancy features in solid tumor models. With the antecedents presented in this bibliographic report, we want to strengthen the idea that the Hv1 proton channel is an excellent therapeutic strategy to counter the development of solid tumors.
Keywords: Hv1; cancer; channel; metastasis (cancer metastasis); oncology; pH; proton; tumor.
Copyright © 2023 Alvear-Arias, Pena-Pichicoi, Carrillo, Fernandez, Gonzalez, Garate and Gonzalez.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Alvear-Arias J. J., Carrillo C., Villar J. P., Garcia-Betancourt R., Peña-Pichicoi A., Fernandez A., et al. (2022). Expression of H v 1 proton channels in myeloid-derived suppressor cells (MDSC) and its potential role in T cell regulation. Proc. Natl. Acad. Sci. 119, e2104453119. 10.1073/pnas.2104453119 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

