Reconstitution and post-thaw storage of cryopreserved human mesenchymal stromal cells: Pitfalls and optimizations for clinically compatible formulants
- PMID: 37153832
- PMCID: PMC10154666
- DOI: 10.1016/j.reth.2023.03.006
Reconstitution and post-thaw storage of cryopreserved human mesenchymal stromal cells: Pitfalls and optimizations for clinically compatible formulants
Abstract
Introduction: The regenerative and immunomodulatory properties of multipotent mesenchymal stromal cells (MSCs) make them an intriguing asset for therapeutic applications. An off-the-shelf approach, using pre-expanded cryopreserved allogenic MSCs, bypasses many practical difficulties of cellular therapy. Reconstitution of a MSC product away from cytotoxic cryoprotectants towards a preferred administration solution might be favorable for several indications. Variations in MSC handling accompanied by a non-standardized use of reconstitution solutions complicate a general clinical standardization of MSC cellular therapies. In this study, we aimed to identify a simple and clinically compatible approach for thawing, reconstitution, and post-thaw storage of cryopreserved MSCs.
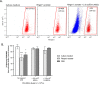
Methods: Human adipose tissue-derived MSCs were expanded in human platelet lysate (hPL) supplemented culture medium and cryopreserved using a dimethyl sulfoxide (DMSO)-based cryoprotectant. Isotonic solutions (saline, Ringer's acetate and phosphate buffered saline (PBS)) with or without 2% human serum albumin (HSA) were used as thawing, reconstitution, and storage solutions. MSCs were reconstituted to 5 × 106 MSCs/mL for evaluating MSC stability. Total MSC numbers and viability were determined using 7-aminoactinomycin D (7-AAD) and flow cytometry.
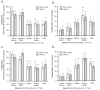
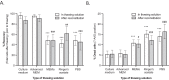
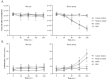
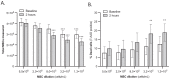
Results: For thawing cryopreserved MSCs the presence of protein was proven to be essential. Up to 50% of MSCs were lost when protein-free thawing solutions were used. Reconstitution and post-thaw storage of MSCs in culture medium and widely used PBS demonstrated poor MSC stability (>40% cell loss) and viability (<80%) after 1 h of storage at room temperature. Reconstitution in simple isotonic saline appeared to be a good alternative for post-thaw storage, ensuring >90% viability with no observed cell loss for at least 4 h. Reconstitution of MSCs to low concentrations was identified as critical. Diluting MSCs to <105/mL in protein-free vehicles resulted in instant cell loss (>40% cell loss) and lower viability (<80%). Addition of clinical grade HSA could prevent cell loss during thawing and dilution.
Conclusion: This study identified a clinically compatible method for MSC thawing and reconstitution that ensures high MSC yield, viability, and stability. The strength of the method lies within the simplicity of implementation which offers an accessible way to streamline MSC therapies across different laboratories and clinical trials, improving standardization in this field.
Keywords: Cell viability; Cellular therapy; Cryopreservation; Mesenchymal stromal cells; Reconstitution; Thawing.
© 2023 The Japanese Society for Regenerative Medicine. Production and hosting by Elsevier B.V.
Conflict of interest statement
All the authors declare no conflict of interest.
Figures


References
-
- Uccelli A., Moretta L., Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8(9):726–736. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

