Sutimlimab for the Treatment of Cold Agglutinin Disease
- PMID: 37153870
- PMCID: PMC10155901
- DOI: 10.1097/HS9.0000000000000879
Sutimlimab for the Treatment of Cold Agglutinin Disease
Abstract
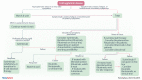
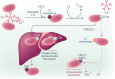
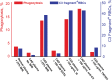
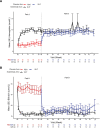
Cold agglutinin disease (CAD) is a rare autoimmune hemolytic anemia and a bone marrow clonal lymphoproliferative disorder. Hemolysis in CAD is complement-dependent and mediated by the classical activation pathway. Patients also frequently suffer from fatigue and cold-induced circulatory symptoms. Although not all patients need treatment, the symptom burden has previously been underestimated. Effective therapies target the clonal lymphoproliferation or the complement activation. Sutimlimab, a humanized monoclonal IgG4 antibody that binds and inactivates complement protein C1s, is the most extensively investigated complement inhibitor for the treatment of CAD. This review addresses the preclinical studies of sutimlimab and the studies of pharmacokinetics and pharmacodynamics. We then describe and discuss the prospective clinical trials that established sutimlimab as a rapidly acting, highly efficacious, and low-toxic therapeutic agent. This complement inhibitor does not improve the cold-induced circulatory symptoms, which are not complement-mediated. Sutimlimab is approved for the treatment of CAD in the US, Japan, and the European Union. A tentative therapeutic algorithm is presented. The choice of therapy for CAD should be based on an individual assessment, and patients requiring therapy should be considered for inclusion in clinical trials.
Copyright © 2023 the Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the European Hematology Association.
Conflict of interest statement
Outside this work, SB has received advisory board honoraria from Anexon, Momenta Pharmaceuticals, Sanofi-Genzyme, and Sobi, and lecture honoraria from Apellis, BeiGene, Janssen-Cilag, Sanofi-Genzyme, and Sobi. The author has no conflicts of interest to disclose.
Figures
References
-
- Michel M. Classification and therapeutic approaches in autoimmune hemolytic anemia: an update. Expert Rev Hematol. 2011;4:607–618. - PubMed
-
- Jäger U, Barcellini W, Broome CM, et al. . Diagnosis and treatment of autoimmune hemolytic anemia in adults: recommendations from the First International Consensus Meeting. Blood Rev. 2020;41:100648. - PubMed
-
- Berentsen S. How I treat cold agglutinin disease. Blood. 2021;137:1295–1303. - PubMed
-
- Berentsen S, Barcellini W. Autoimmune hemolytic anemias. N Engl J Med. 2021;385:1407–1419. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
