NK Cell Phenotype Is Associated With Response and Resistance to Daratumumab in Relapsed/Refractory Multiple Myeloma
- PMID: 37153876
- PMCID: PMC10155898
- DOI: 10.1097/HS9.0000000000000881
NK Cell Phenotype Is Associated With Response and Resistance to Daratumumab in Relapsed/Refractory Multiple Myeloma
Abstract
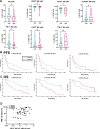
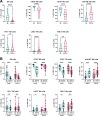
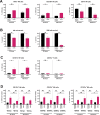
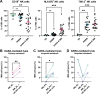
The CD38-targeting antibody daratumumab has marked activity in multiple myeloma (MM). Natural killer (NK) cells play an important role during daratumumab therapy by mediating antibody-dependent cellular cytotoxicity via their FcγRIII receptor (CD16), but they are also rapidly decreased following initiation of daratumumab treatment. We characterized the NK cell phenotype at baseline and during daratumumab monotherapy by flow cytometry and cytometry by time of flight to assess its impact on response and development of resistance (DARA-ATRA study; NCT02751255). At baseline, nonresponding patients had a significantly lower proportion of CD16+ and granzyme B+ NK cells, and higher frequency of TIM-3+ and HLA-DR+ NK cells, consistent with a more activated/exhausted phenotype. These NK cell characteristics were also predictive of inferior progression-free survival and overall survival. Upon initiation of daratumumab treatment, NK cells were rapidly depleted. Persisting NK cells exhibited an activated and exhausted phenotype with reduced expression of CD16 and granzyme B, and increased expression of TIM-3 and HLA-DR. We observed that addition of healthy donor-derived purified NK cells to BM samples from patients with either primary or acquired daratumumab-resistance improved daratumumab-mediated MM cell killing. In conclusion, NK cell dysfunction plays a role in primary and acquired daratumumab resistance. This study supports the clinical evaluation of daratumumab combined with adoptive transfer of NK cells.
Copyright © 2023 the Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the European Hematology Association.
Conflict of interest statement
MCM has a consultancy or advisory role for Gilead Sciences, BMS, Alnylam, Janssen Cilag, Takeda, and Servier; all paid to employer, hospitality from Celgene. MDL serves in advisory boards for Roche, Janssen and Abbvia. AB receives honoraria from Celgene, Janssen, Amgen, Takeda, and Sanofi. MJK has received research support from Kite/Gilead, and honoraria from Kite/Gilead, Novartis, BMS/Celgene, Takeda, Roche, and Miltenyi Biotec (all paid to institution). MK, TC, YA, RIV, TS, GV, DCS, LvS, and ER are employed by Janssen. PS has received honoraria from Amgen, BMS, Celgene, Janssen, Karyopharm, Takeda, and receives research funding from Amgen, Celgene, Janssen, Karyopharm, SkylineDx, Takeda. SZ has received research funding from Celgene, Takeda, Janssen, and serves in advisory boards for Janssen, Takeda, BMS, Oncopeptides and Sanofi, all paid to institution. TM has received research support from Janssen Pharmaceuticals, Takeda, Genmab, Novartis, and ONK Therapeutics. NWCJvdD has received research support from Janssen Pharmaceuticals, AMGEN, Celgene, Novartis, Cellectis, and BMS, and serves in advisory boards for Janssen Pharmaceuticals, AMGEN, Celgene, BMS, Takeda, Roche, Novartis, Bayer, Adaptive, and Servier. All the other authors have no conflicts of interest to disclose.
Figures



References
-
- van de Donk N, Richardson PG, Malavasi F. CD38 antibodies in multiple myeloma: back to the future. Blood. 2018;131:13–29. - PubMed
-
- Lammerts van Bueren J, Jakobs D, Kaldenhoven N, et al. . Direct in Vitro comparison of daratumumab with surrogate analogs of CD38 antibodies MOR03087, SAR650984 and Ab79. Blood. 2014;124:3474–3474.
-
- Van De Donk NWCJ, Adams H, Vanhoof G, et al. . Daratumumab in Combination with Lenalidomide Plus Dexamethasone Results in Persistent Natural Killer (NK) Cells with a Distinct Phenotype and Expansion of Effector Memory T-Cells in Pollux, a Phase 3 Randomized Study. Blood. 2017;130(Suppl 1):3124. http://www.bloodjournal.org/content/130/Suppl_1/3124.abstract.
LinkOut - more resources
Full Text Sources
Research Materials
