Acrylamide induces human chondrocyte cell death by initiating autophagy‑dependent ferroptosis
- PMID: 37153903
- PMCID: PMC10160918
- DOI: 10.3892/etm.2023.11945
Acrylamide induces human chondrocyte cell death by initiating autophagy‑dependent ferroptosis
Abstract
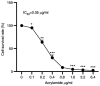
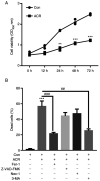
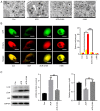
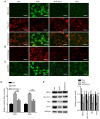
Acrylamide (ACR) is formed during heat treatment of foodstuffs and ACR may serve as a probable malignant neoplastic disease agent in all organs and tissues of the human body. However, it is unknown if ACR is associated with ankylosing spondylitis (AS) pathogenesis. Cell viability and proliferation were determined using CCK-8 assay and EdU staining. Flow cytometry was used to determine cell death and cell cycle arrest. Intracellular lipid reactive oxygen species, Fe2+ and mitochondrial membrane potential (MMP) were analyzed using a C11-BODIPY581/591 fluorescent probe, FerroOrange staining and a JC-1 MMP Assay kit, respectively. The present study showed that ACR decreased chondrocyte cell viability in a dose-dependent manner and that ACR significantly promoted chondrocyte senescence. ACR also elevated the expression of cell cycle arrest-associated proteins, including p53, cyclin-dependent kinase inhibitor 1 and cyclin-dependent kinase inhibitor protein, in human chondrocytes. Similarly, DNA damage was also enhanced following ACR treatment in chondrocytes. In addition, the ferroptosis-specific inhibitor ferrostatin-1 (Fer-1) and the autophagy inhibitor 3-methyladenine abolished ACR-induced cell death in chondrocytes. ACR was shown to activate autophagic flux and induce mitochondrial dysfunction by increasing the MMP. Western blot analysis of ferroptosis-related proteins demonstrated that ACR decreased the expression of glutathione peroxidase 4, solute carrier family 7 member 11, transferrin receptor protein 1 and ferritin heavy chain 1 in chondrocytes whereas Fer-1 abolished these effects. ACR treatment significantly elevated the phosphorylation levels of AMP-activated protein kinase (AMPK) and serine/threonine-protein kinase ULK1 in human chondrocytes. Notably, the effect of ACR was diminished by knockdown of AMPK, as evidenced by reduced lipid reactive oxygen species accumulation and Fe2+ levels. Hence, ACR inhibited cell proliferation and contributed to cell death by inducing autophagy-dependent ferroptosis while promoting autophagy by activating AMPK-ULK1-mTOR signaling in human chondrocytes. It was hypothesized that the presence of ACR in foodstuffs may increase the risk of AS and that decreasing ACR in food products is of importance.
Keywords: AMPK/ULK1/mTOR signaling; acrylamide; ankylosing spondylitis; autophagy; ferroptosis.
Copyright © 2020, Spandidos Publications.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Activating autophagy and ferroptosis of 3‑Chloropropane‑1,2‑diol induces injury of human umbilical vein endothelial cells via AMPK/mTOR/ULK1.Mol Med Rep. 2023 Mar;27(3):76. doi: 10.3892/mmr.2023.12963. Epub 2023 Feb 17. Mol Med Rep. 2023. Retraction in: Mol Med Rep. 2025 Jan;31(1):12. doi: 10.3892/mmr.2024.13377. PMID: 36799162 Free PMC article. Retracted.
-
Amentoflavone suppresses cell proliferation and induces cell death through triggering autophagy-dependent ferroptosis in human glioma.Life Sci. 2020 Apr 15;247:117425. doi: 10.1016/j.lfs.2020.117425. Epub 2020 Feb 11. Life Sci. 2020. Retraction in: Life Sci. 2024 May 1;344:122588. doi: 10.1016/j.lfs.2024.122588. PMID: 32057904 Retracted.
-
Ferritinophagy is involved in the zinc oxide nanoparticles-induced ferroptosis of vascular endothelial cells.Autophagy. 2021 Dec;17(12):4266-4285. doi: 10.1080/15548627.2021.1911016. Epub 2021 Apr 12. Autophagy. 2021. PMID: 33843441 Free PMC article.
-
Dihydroartemisinin eliminates senescent cells by promoting autophagy-dependent ferroptosis via AMPK/mTOR signaling pathway.Cell Biol Int. 2024 May;48(5):726-736. doi: 10.1002/cbin.12143. Epub 2024 Mar 4. Cell Biol Int. 2024. PMID: 38439187
-
SIRT3 deficiency is resistant to autophagy-dependent ferroptosis by inhibiting the AMPK/mTOR pathway and promoting GPX4 levels.J Cell Physiol. 2020 Nov;235(11):8839-8851. doi: 10.1002/jcp.29727. Epub 2020 Apr 24. J Cell Physiol. 2020. PMID: 32329068
Cited by
-
Acrylamide and Its Metabolite Glycidamide Induce Reproductive Toxicity During In Vitro Maturation of Bovine Oocytes.Toxics. 2025 Mar 19;13(3):223. doi: 10.3390/toxics13030223. Toxics. 2025. PMID: 40137550 Free PMC article.
-
Iron metabolism and arthritis: Exploring connections and therapeutic avenues.Chin Med J (Engl). 2024 Jul 20;137(14):1651-1662. doi: 10.1097/CM9.0000000000003169. Epub 2024 Jun 12. Chin Med J (Engl). 2024. PMID: 38867424 Free PMC article. Review.
-
Decoding ferroptosis: transforming orthopedic disease management.Front Pharmacol. 2024 Dec 6;15:1509172. doi: 10.3389/fphar.2024.1509172. eCollection 2024. Front Pharmacol. 2024. PMID: 39712490 Free PMC article. Review.
References
-
- Steiner M, Del Mar Esteban-Ortega M, Thuissard-Vasallo I, García-Lozano I, Moriche-Carretero M, García-González AJ, Pérez-Blázquez E, Sambricio J, García-Aparicio Á, Casco-Silva BF, et al. Measuring choroid thickness as a marker of systemic inflammation in patients with ankylosing spondylitis. J Clin Rheumatol. 2021;27:e307–e311. doi: 10.1097/RHU.0000000000001348. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
