Identification of food and nutrient components as predictors of Lactobacillus colonization
- PMID: 37153913
- PMCID: PMC10160632
- DOI: 10.3389/fnut.2023.1118679
Identification of food and nutrient components as predictors of Lactobacillus colonization
Abstract
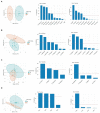
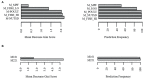
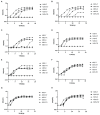
A previous double-blind, randomized clinical trial of 42 healthy individuals conducted with Lactobacillus johnsonii N6.2 found that the probiotic's mechanistic tryptophan pathway was significantly modified when the data was stratified based on the individuals' lactic acid bacteria (LAB) stool content. These results suggest that confounding factors such as dietary intake which impact stool LAB content may affect the response to the probiotic treatment. Using dietary intake, serum metabolite, and stool LAB colony forming unit (CFU) data from a previous clinical trial, the relationships between diet, metabolic response, and fecal LAB were assessed. The diets of subject groups with high vs. low CFUs of LAB/g of wet stool differed in their intakes of monounsaturated fatty acids, vegetables, proteins, and dairy. Individuals with high LAB consumed greater amounts of cheese, fermented meats, soy, nuts and seeds, alcoholic beverages, and oils whereas individuals with low LAB consumed higher amounts of tomatoes, starchy vegetables, and poultry. Several dietary variables correlated with LAB counts; positive correlations were determined for nuts and seeds, fish high in N-3 fatty acids, soy, and processed meats, and negative correlations to consumption of vegetables including tomatoes. Using machine learning, predictors of LAB count included cheese, nuts and seeds, fish high in N-3 fatty acids, and erucic acid. Erucic acid alone accurately predicted LAB categorization, and was shown to be utilized as a sole fatty acid source by several Lactobacillus species regardless of their mode of fermentation. Several metabolites were significantly upregulated in each group based on LAB titers, notably polypropylene glycol, caproic acid, pyrazine, and chondroitin sulfate; however, none were correlated with the dietary intake variables. These findings suggest that dietary variables may drive the presence of LAB in the human gastrointestinal tract and potentially impact response to probiotic interventions.
Keywords: Lactobacillus johnsonii N6.2; dietary intake; lactic acid bacteria; machine learning; microbiome; multivariate analysis; probiotic.
Copyright © 2023 Thompson, Ford, Moothedan, Stafford, Garrett, Dahl, Conesa, Gonzalez and Lorca.
Conflict of interest statement
GL holds U.S. patent No. 9,474,773 and 9,987,313 on Lactobacillus johnsonii N6.2. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Figures
References
-
- Wold AE. Immune effects of probiotics: Näringsforskning. Food Nutr. (2001) 45:76–85. doi: 10.3402/fnr.v45i0.1787 - DOI
-
- Alyousif Z, Miller JL, Auger J, Sandoval M, Piano A, Tompkins TA, et al. . Microbiota profile and efficacy of probiotic supplementation on laxation in adults affected by Prader-Willi syndrome: a randomized, double-blind, crossover trial. Mol Gene Geno Med. (2020) 8:e1535. doi: 10.1002/mgg3.1535, PMID: - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

