Calcium-binding properties, stability, and osteogenic ability of phosphorylated soy peptide-calcium chelate
- PMID: 37153921
- PMCID: PMC10160607
- DOI: 10.3389/fnut.2023.1129548
Calcium-binding properties, stability, and osteogenic ability of phosphorylated soy peptide-calcium chelate
Abstract
Introduction: Bioactive peptides based on foodstuffs are of particular interest as carriers for calcium delivery due to their safety and high activity. The phosphorylated peptide has been shown to enhance calcium absorption and bone formation.
Method: A novel complex of peptide phosphorylation modification derived from soybean protein was introduced, and the mechanism, stability, and osteogenic differentiation bioactivity of the peptide with or without calcium were studied.
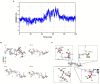
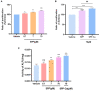
Result: The calcium-binding capacity of phosphorylated soy peptide (SPP) reached 50.24 ± 0.20 mg/g. The result of computer stimulation and vibration spectrum showed that SPP could chelate with calcium by the phosphoric acid group, carboxyl oxygen of C-terminal Glu, Asp, and Arg, and phosphoric acid group of Ser on the SPP at a stoichiometric ratio of 1:1, resulting in the formation of the complex of ligand and peptide. Thermal stability showed that chelation enhanced peptide stability compared with SPP alone. Additionally, in vitro results showed that SPP-Ca could facilitate osteogenic proliferation and differentiation ability.
Discussion: SPP may function as a promising alternative to current therapeutic agents for bone loss.
Keywords: calcium supplement; characterization; osteogenic differentiation; peptide-calcium chelate; phosphorylation; thermal stability.
Copyright © 2023 Kong, Xiao, Chen, Du, Zhang, Wang, Xu, Cheng, Yu and Gan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Guo L, Harnedy PA, Li B, Hou H, Zhang Z, Zhao X, et al. . Food protein-derived chelating peptides: Biofunctional ingredients for dietary mineral bioavailability enhancement. Trends Food Sci Technol. (2014) 37:92–105. 10.1016/j.tifs.2014.02.007 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

