Downregulation of FKBP5 Promotes Atrial Arrhythmogenesis
- PMID: 37154033
- PMCID: PMC10330339
- DOI: 10.1161/CIRCRESAHA.122.322213
Downregulation of FKBP5 Promotes Atrial Arrhythmogenesis
Abstract
Background: Atrial fibrillation (AF), the most common arrhythmia, is associated with the downregulation of FKBP5 (encoding FKBP5 [FK506 binding protein 5]). However, the function of FKBP5 in the heart remains unknown. Here, we elucidate the consequences of cardiomyocyte-restricted loss of FKBP5 on cardiac function and AF development and study the underlying mechanisms.
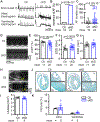
Methods: Right atrial samples from patients with AF were used to assess the protein levels of FKBP5. A cardiomyocyte-specific FKBP5 knockdown mouse model was established by crossbreeding Fkbp5flox/flox mice with Myh6MerCreMer/+ mice. Cardiac function and AF inducibility were assessed by echocardiography and programmed intracardiac stimulation. Histology, optical mapping, cellular electrophysiology, and biochemistry were employed to elucidate the proarrhythmic mechanisms due to loss of cardiomyocyte FKBP5.
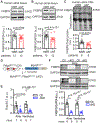
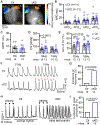
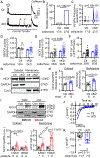
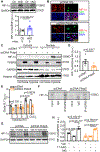
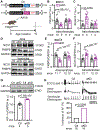
Results: FKBP5 protein levels were lower in the atrial lysates of patients with paroxysmal AF or long-lasting persistent (chronic) AF. Cardiomyocyte-specific knockdown mice exhibited increased AF inducibility and duration compared with control mice. Enhanced AF susceptibility in cardiomyocyte-specific knockdown mice was associated with the development of action potential alternans and spontaneous Ca2+ waves, and increased protein levels and activity of the NCX1 (Na+/Ca2+-exchanger 1), mimicking the cellular phenotype of chronic AF patients. FKBP5-deficiency enhanced transcription of Slc8a1 (encoding NCX1) via transcription factor hypoxia-inducible factor 1α. In vitro studies revealed that FKBP5 negatively modulated the protein levels of hypoxia-inducible factor 1α by competitively interacting with heat-shock protein 90. Injections of the heat-shock protein 90 inhibitor 17-AAG normalized protein levels of hypoxia-inducible factor 1α and NCX1 and reduced AF susceptibility in cardiomyocyte-specific knockdown mice. Furthermore, the atrial cardiomyocyte-selective knockdown of FKBP5 was sufficient to enhance AF arrhythmogenesis.
Conclusions: This is the first study to demonstrate a role for the FKBP5-deficiency in atrial arrhythmogenesis and to establish FKBP5 as a negative regulator of hypoxia-inducible factor 1α in cardiomyocytes. Our results identify a potential molecular mechanism for the proarrhythmic NCX1 upregulation in chronic AF patients.
Keywords: animals; atrial fibrillation; electrophysiology; mice.
Conflict of interest statement
Figures


Comment in
-
A Novel Role for FKBP5 in Atrial Cardiomyopathy.Circ Res. 2023 Jun 23;133(1):45-47. doi: 10.1161/CIRCRESAHA.123.322988. Epub 2023 Jun 22. Circ Res. 2023. PMID: 37347835 No abstract available.
References
-
- Zannas AS, Balsevich G and Gassen NC. The emerging role of FKBP5 in the regulation of metabolism and body weight. Surg Obes Relat Dis. 2016;12:1560–1561. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

