Long-Term Safety and Efficacy of Elexacaftor/Tezacaftor/Ivacaftor in Children Aged ⩾6 Years with Cystic Fibrosis and at Least One F508del Allele: A Phase 3, Open-Label Clinical Trial
- PMID: 37154609
- PMCID: PMC10870850
- DOI: 10.1164/rccm.202301-0021OC
Long-Term Safety and Efficacy of Elexacaftor/Tezacaftor/Ivacaftor in Children Aged ⩾6 Years with Cystic Fibrosis and at Least One F508del Allele: A Phase 3, Open-Label Clinical Trial
Abstract
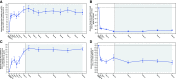
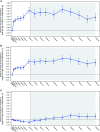
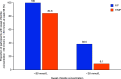
Rationale: A 24-week, phase 3, open-label study showed elexacaftor/tezacaftor/ivacaftor (ELX/TEZ/IVA) was safe and efficacious in children aged 6-11 years with cystic fibrosis (CF) and one or more F508del-CFTR alleles. Objectives: To assess long-term safety and efficacy of ELX/TEZ/IVA in children who completed the pivotal 24-week phase 3 trial. Methods: In this phase 3, two-part (part A and part B), open-label extension study, children aged ⩾6 years with CF heterozygous for F508del and a minimal function CFTR mutation (F/MF genotypes) or homozygous for F508del (F/F genotype) who completed the 24-week parent study received ELX/TEZ/IVA based on weight. Children weighing <30 kg received ELX 100 mg once daily/TEZ 50 mg once daily/IVA 75 mg every 12 hours, whereas children weighing ⩾30 kg received ELX 200 mg once daily/TEZ 100 mg once daily/IVA 150 mg every 12 hours (adult dose). The 96-week analysis of part A of this extension study is reported here. Measurements and Main Results: Sixty-four children (F/MF genotypes, n = 36; F/F genotype, n = 28) were enrolled and received one or more doses of ELX/TEZ/IVA. Mean (SD) period of exposure to ELX/TEZ/IVA was 93.9 (11.1) weeks. The primary endpoint was safety and tolerability. Adverse events and serious adverse events were consistent with common manifestations of CF disease. Overall, exposure-adjusted rates of adverse events and serious adverse events (407.74 and 4.72 events per 100 patient-years) were lower than in the parent study (987.04 and 8.68 events per 100 patient-years). One child (1.6%) had an adverse event of aggression that was moderate in severity and resolved after study drug discontinuation. From parent study baseline at Week 96 of this extension study, the mean percent predicted FEV1 increased (11.2 [95% confidence interval (CI), 8.3 to 14.2] percentage points), sweat chloride concentration decreased (-62.3 [95% CI, -65.9 to -58.8] mmol/L), Cystic Fibrosis Questionnaire-Revised respiratory domain score increased (13.3 [95% CI, 11.4 to 15.1] points), and lung clearance index 2.5 decreased (-2.00 [95% CI, -2.45 to -1.55] units). Increases in growth parameters were also observed. The estimated pulmonary exacerbation rate per 48 weeks was 0.04. The annualized rate of change in percent predicted FEV1 was 0.51 (95% CI, -0.73 to 1.75) percentage points per year. Conclusions: ELX/TEZ/IVA continued to be generally safe and well tolerated in children aged ⩾6 years through an additional 96 weeks of treatment. Improvements in lung function, respiratory symptoms, and CFTR function observed in the parent study were maintained. These results demonstrate the favorable long-term safety profile and durable clinical benefits of ELX/TEZ/IVA in this pediatric population. Clinical trial registered with www.clinicaltrials.gov (NCT04183790).
Keywords: CFTR modulator; children; elexacaftor/tezacaftor/ivacaftor; long-term extension study.
Figures

Comment in
-
Cystic Fibrosis: From Tragedy to Triumph.Am J Respir Crit Care Med. 2023 Jul 1;208(1):9-11. doi: 10.1164/rccm.202305-0785ED. Am J Respir Crit Care Med. 2023. PMID: 37167625 Free PMC article. No abstract available.
References
-
- Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science . 1989;245:1066–1073. - PubMed
-
- VanDevanter DR, Kahle JS, O’Sullivan AK, Sikirica S, Hodgkins PS. Cystic fibrosis in young children: a review of disease manifestation, progression, and response to early treatment. J Cyst Fibros . 2016;15:147–157. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

