Generation of a novel three-dimensional scaffold-based model of the bovine endometrium
- PMID: 37154859
- PMCID: PMC10484811
- DOI: 10.1007/s11259-023-10130-0
Generation of a novel three-dimensional scaffold-based model of the bovine endometrium
Abstract
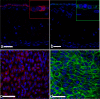
Bovine in vitro endometrial models that resemble tissue function in vivo are needed to study infertility, long-term uterine alterations induced by pathogens and impact of endocrine disruptor chemicals on reproductive function and other reproductive system complications that cause high economic losses in livestock species. The present study aimed to generate an innovative, reproducible, and functional 3D scaffold-based model of the bovine endometrium structurally robust for long term-culture. We developed a multicellular model containing both endometrial epithelial and stromal cells. Epithelial cells organized to form a luminal-like epithelial layer on the surface of the scaffold. Stromal cells produced their own extracellular matrix forming a stable subepithelial compartment that physiologically resembles the normal endometrium. Both cell types released prostaglandin E2 and prostaglandin F2α following a treatment with oxytocin and arachidonic acid. Additionally signal pathways mediating oxytocin and arachidonic acid stimulation of prostaglandin synthesis were analyzed by real time PCR (RT-PCR). Oxytocin receptor (OXTR), prostaglandin E2 receptor 2 (EP2), prostaglandin E2 receptor 4 (EP4), prostaglandin F receptor (PTGFR), prostaglandin E synthase (PTGES), PGF-synthase (PGFS) and prostaglandin-endoperoxide synthase 2 (COX-2) expression was detected in both control and treatment groups, however, only significant changes in abundance of OXTR mRNA transcripts were found. The results obtained by this study are a step forward in bovine in vitro culture technology. This 3D scaffold-based model provides a platform to study regulatory mechanisms involved in endometrial physiology and can set the basis for a broader tool for designing and testing novel therapeutic strategies for recurrent uterine pathologies.
Keywords: Co-culture; Endometrium; Scaffold; Three-dimensional (3D) cell culture.
© 2023. The Author(s).
Conflict of interest statement
SP acts as a consultant for Reprocell Europe.
Figures








References
-
- Chankeaw W, Lignier S, Richard C, Ntallaris T, Raliou M, Guo Y, Plassard D, Bevilacqua C, Sandra O, Andersson G, Humblot P, Charpigny G. Analysis of the transcriptome of bovine endometrial cells isolated by laser micro-dissection (1): specific signatures of stromal, glandular and luminal epithelial cells. BMC Genomics. 2021;22(1):451. doi: 10.1186/s12864-021-07712-0. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

