Effectiveness of Genotype-Specific Tricyclic Antidepressant Dosing in Patients With Major Depressive Disorder: A Randomized Clinical Trial
- PMID: 37155164
- PMCID: PMC10167565
- DOI: 10.1001/jamanetworkopen.2023.12443
Effectiveness of Genotype-Specific Tricyclic Antidepressant Dosing in Patients With Major Depressive Disorder: A Randomized Clinical Trial
Abstract
Importance: Evidence of the clinical benefit of pharmacogenetics-informed treatment (PIT) with antidepressants is still limited. Especially for tricyclic antidepressants (TCAs), pharmacogenetics may be of interest because therapeutic plasma concentrations are well defined, identification of optimal dosing can be time consuming, and treatment is frequently accompanied by adverse effects.
Objective: To determine whether PIT results in faster attainment of therapeutic TCA plasma concentrations compared with usual treatment in patients with unipolar major depressive disorder (MDD).
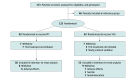
Design, setting, and participants: This randomized clinical trial compared PIT with usual treatment among 111 patients at 4 centers in the Netherlands. Patients were treated with the TCAs nortriptyline, clomipramine, or imipramine, with clinical follow-up of 7 weeks. Patients were enrolled from June 1, 2018, to January 1, 2022. At inclusion, patients had unipolar nonpsychotic MDD (with a score of ≥19 on the 17-item Hamilton Rating Scale for Depression [HAMD-17]), were aged 18 to 65 years, and were eligible for TCA treatment. Main exclusion criteria were a bipolar or psychotic disorder, substance use disorder, pregnancy, interacting comedications, and concurrent use of psychotropic medications.
Intervention: In the PIT group, the initial TCA dosage was based on CYP2D6 and CYP2C19 genotypes. The control group received usual treatment, which comprised the standard initial TCA dosage.
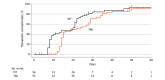
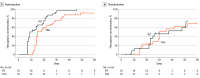
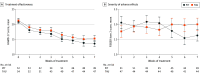
Main outcomes and measures: The primary outcome was days until attainment of a therapeutic TCA plasma concentration. Secondary outcomes were severity of depressive symptoms (measured by HAMD-17 scores) and frequency and severity of adverse effects (measured by Frequency, Intensity, and Burden of Side Effects Rating scores).
Results: Of 125 patients randomized, 111 (mean [SD] age, 41.7 [13.3] years; 69 [62.2%] female) were included in the analysis; of those, 56 were in the PIT group and 55 were in the control group. The PIT group reached therapeutic concentrations faster than the control group (mean [SD], 17.3 [11.2] vs 22.0 [10.2] days; Kaplan-Meier χ21 = 4.30; P = .04). No significant difference in reduction of depressive symptoms was observed. Linear mixed-model analyses showed that the interaction between group and time differed for the frequency (F6,125 = 4.03; P = .001), severity (F6,114 = 3.10; P = .008), and burden (F6,112 = 2.56; P = .02) of adverse effects, suggesting that adverse effects decreased relatively more for those receiving PIT.
Conclusions and relevance: In this randomized clinical trial, PIT resulted in faster attainment of therapeutic TCA concentrations, with potentially fewer and less severe adverse effects. No effect on depressive symptoms was observed. These findings indicate that pharmacogenetics-informed dosing of TCAs can be safely applied and may be useful in personalizing treatment for patients with MDD.
Trial registration: ClinicalTrials.gov Identifier: NCT03548675.
Conflict of interest statement
Figures