Clinical Effect of Early vs Late Amyloid Positron Emission Tomography in Memory Clinic Patients: The AMYPAD-DPMS Randomized Clinical Trial
- PMID: 37155177
- PMCID: PMC10167601
- DOI: 10.1001/jamaneurol.2023.0997
Clinical Effect of Early vs Late Amyloid Positron Emission Tomography in Memory Clinic Patients: The AMYPAD-DPMS Randomized Clinical Trial
Abstract
Importance: Amyloid positron emission tomography (PET) allows the direct assessment of amyloid deposition, one of the main hallmarks of Alzheimer disease. However, this technique is currently not widely reimbursed because of the lack of appropriately designed studies demonstrating its clinical effect.
Objective: To assess the clinical effect of amyloid PET in memory clinic patients.
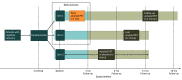
Design, setting, and participants: The AMYPAD-DPMS is a prospective randomized clinical trial in 8 European memory clinics. Participants were allocated (using a minimization method) to 3 study groups based on the performance of amyloid PET: arm 1, early in the diagnostic workup (within 1 month); arm 2, late in the diagnostic workup (after a mean [SD] 8 [2] months); or arm 3, if and when the managing physician chose. Participants were patients with subjective cognitive decline plus (SCD+; SCD plus clinical features increasing the likelihood of preclinical Alzheimer disease), mild cognitive impairment (MCI), or dementia; they were assessed at baseline and after 3 months. Recruitment took place between April 16, 2018, and October 30, 2020. Data analysis was performed from July 2022 to January 2023.
Intervention: Amyloid PET.
Main outcome and measure: The main outcome was the difference between arm 1 and arm 2 in the proportion of participants receiving an etiological diagnosis with a very high confidence (ie, ≥90% on a 50%-100% visual numeric scale) after 3 months.
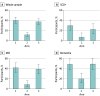
Results: A total of 844 participants were screened, and 840 were enrolled (291 in arm 1, 271 in arm 2, 278 in arm 3). Baseline and 3-month visit data were available for 272 participants in arm 1 and 260 in arm 2 (median [IQR] age: 71 [65-77] and 71 [65-77] years; 150/272 male [55%] and 135/260 male [52%]; 122/272 female [45%] and 125/260 female [48%]; median [IQR] education: 12 [10-15] and 13 [10-16] years, respectively). After 3 months, 109 of 272 participants (40%) in arm 1 had a diagnosis with very high confidence vs 30 of 260 (11%) in arm 2 (P < .001). This was consistent across cognitive stages (SCD+: 25/84 [30%] vs 5/78 [6%]; P < .001; MCI: 45/108 [42%] vs 9/102 [9%]; P < .001; dementia: 39/80 [49%] vs 16/80 [20%]; P < .001).
Conclusion and relevance: In this study, early amyloid PET allowed memory clinic patients to receive an etiological diagnosis with very high confidence after only 3 months compared with patients who had not undergone amyloid PET. These findings support the implementation of amyloid PET early in the diagnostic workup of memory clinic patients.
Trial registration: EudraCT Number: 2017-002527-21.
Conflict of interest statement
Figures


References
-
- Chiotis K, Saint-Aubert L, Boccardi M, et al. ; Geneva Task Force for the Roadmap of Alzheimer’s Biomarkers . Clinical validity of increased cortical uptake of amyloid ligands on PET as a biomarker for Alzheimer’s disease in the context of a structured 5-phase development framework. Neurobiol Aging. 2017;52:214-227. doi:10.1016/j.neurobiolaging.2016.07.012 - DOI - PubMed
-
- Boccardi M, Altomare D, Ferrari C, et al. ; Incremental Diagnostic Value of Amyloid PET With [18F]-Florbetapir (INDIA-FBP) Working Group . Assessment of the incremental diagnostic value of florbetapir F 18 imaging in patients with cognitive impairment: the Incremental Diagnostic Value of Amyloid PET With [18F]-Florbetapir (INDIA-FBP) Study. JAMA Neurol. 2016;73(12):1417-1424. doi:10.1001/jamaneurol.2016.3751 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

