Clinical outcomes of cemented distal femur replacements with all-polyethylene tibial components for oncologic indications
- PMID: 37155507
- PMCID: PMC10122776
- DOI: 10.5312/wjo.v14.i4.218
Clinical outcomes of cemented distal femur replacements with all-polyethylene tibial components for oncologic indications
Abstract
Background: Endoprosthetic distal femoral replacement (DFR) is a well-established salvage procedure following resection of malignant tumors within the distal femur. Use of an all-polyethylene tibial (APT) component is cost-effective and avoids failure due to locking-mechanism issues and backside wear, but limits modularity and the option for late liner exchange. Due to a paucity of literature we sought to answer three questions: (1) What are the most common modes of implant failure for patients undergoing cemented DFR with APT for oncologic indications? (2) What is the survivorship, rate of all-cause reoperation, and rate of revision for aseptic loosening of these implants? And (3) Is there a difference in implant survivorship or patient demographics between cemented DFRs with APT performed as a primary reconstruction vs those performed as a revision procedure?
Aim: To assess outcomes of cemented DFRs with APT components used for oncologic indications.
Methods: After Institutional Review Board approval, a retrospective review of consecutive patients who underwent DFR between December 2000 to September 2020 was performed using a single-institutional database. Inclusion criteria consisted of all patients who underwent DFR with a GMRS® (Global Modular Replacement System, Stryker, Kalamazoo, MI, United States) cemented distal femoral endoprosthesis and APT component for an oncologic indication. Patients undergoing DFR for non-oncologic indications and patients with metal-backed tibial components were excluded. Implant failure was recorded using Henderson's classification and survivorship was reported using a competing risks analysis.
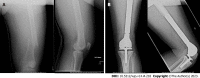
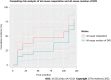
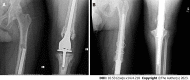
Results: 55 DFRs (55 patients) with an average age of 50.9 ± 20.7 years and average body mass index of 29.7 ± 8.3 kg/m2 were followed for 38.8 ± 54.9 mo (range 0.2-208.4). Of these, 60.0% were female and 52.7% were white. The majority of DFRs with APT in this cohort were indicated for oncologic diagnoses of osteogenic sarcoma (n = 22, 40.0%), giant cell tumor (n = 9, 16.4%), and metastatic carcinoma (n = 8, 14.6%). DFR with APT implantation was performed as a primary procedure in 29 patients (52.7%) and a revision procedure in 26 patients (47.3%). Overall, twenty patients (36.4%) experienced a postoperative complication requiring reoperation. The primary modes of implant failure included Henderson Type 1 (soft tissue failure, n = 6, 10.9%), Type 2 (aseptic loosening, n = 5, 9.1%), and Type 4 (infection, n = 6, 10.9%). There were no significant differences in patient demographics or rates of postoperative complications between the primary procedure and revision procedure subgroups. In total, 12 patients (21.8%) required a revision while 20 patients (36.4%) required a reoperation, resulting in three-year cumulative incidences of 24.0% (95%CI 9.9%-41.4%) and 47.2% (95%CI 27.5%-64.5%), respectively.
Conclusion: This study demonstrates modest short-term survivorship following cemented DFR with APT components for oncologic indications. Soft tissue failure and endoprosthetic infection were the most common postoperative complications in our cohort.
Keywords: Dislocation; Distal femoral replacement; Modular; Oncologic; Revision.
©The Author(s) 2023. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: One of the authors (N.D.H.) is a paid consultant for Intellijoint Surgical and MicroPort Orthopedics, has stock options from Intellijoint Surgical, and is a committee member of AAOS and AJRR. One of the authors (L.R.M.) is a paid consultant for Onkos Surgical and receives IP royalties from TeDan Surgical. Each author certifies that he has no commercial associations that might pose a conflict of interest in connection with the submitted article. One of the authors (A.B.C) is a board member for AAOS, Musculoskeletal Tumor Society, Orthopaedic Society. (A.B.C) is also a paid consultant for Smith and Nephew, Intellijoint Surgical, and Enovis.
Figures

Similar articles
-
What Is the Long-term Survivorship of Primary and Revision Cemented Distal Femoral Replacements for Limb Salvage of Patients With Sarcoma?Clin Orthop Relat Res. 2023 Mar 1;481(3):460-471. doi: 10.1097/CORR.0000000000002333. Epub 2022 Aug 8. Clin Orthop Relat Res. 2023. PMID: 35943730 Free PMC article.
-
Long-Term Results of Total Knee Arthroplasty with Contemporary Distal Femoral Replacement.J Bone Joint Surg Am. 2020 Jan 2;102(1):45-51. doi: 10.2106/JBJS.19.00489. J Bone Joint Surg Am. 2020. PMID: 31596808
-
Is High-dose Radiation Therapy Associated With Early Revision Due to Aseptic Loosening in Patients With a Sarcoma of the Lower Extremities Reconstructed With a Cemented Endoprosthesis?Clin Orthop Relat Res. 2023 Mar 1;481(3):475-487. doi: 10.1097/CORR.0000000000002360. Epub 2022 Aug 17. Clin Orthop Relat Res. 2023. PMID: 35977001 Free PMC article.
-
Implant Survival and Complication Profiles of Endoprostheses for Treating Tumor Around the Knee in Adults: A Systematic Review of the Literature Over the Past 30 Years.J Arthroplasty. 2018 Apr;33(4):1275-1287.e3. doi: 10.1016/j.arth.2017.10.051. Epub 2017 Nov 7. J Arthroplasty. 2018. PMID: 29191444
-
Causes and Frequencies of Reoperations After Endoprosthetic Reconstructions for Extremity Tumor Surgery: A Systematic Review.Clin Orthop Relat Res. 2019 Apr;477(4):894-902. doi: 10.1097/CORR.0000000000000630. Clin Orthop Relat Res. 2019. PMID: 30801278 Free PMC article.
Cited by
-
Assessing the influence of gastrocnemius reconstruction on stress distribution of femoral tumor rotating hinge knee prosthesis via finite element analysis.Front Bioeng Biotechnol. 2024 Apr 19;12:1391298. doi: 10.3389/fbioe.2024.1391298. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38707499 Free PMC article.
References
-
- Simon MA, Aschliman MA, Thomas N, Mankin HJ. Limb-salvage treatment vs amputation for osteosarcoma of the distal end of the femur. J Bone Joint Surg Am. 1986;68:1331–1337. - PubMed
-
- Unwin PS, Cannon SR, Grimer RJ, Kemp HB, Sneath RS, Walker PS. Aseptic loosening in cemented custom-made prosthetic replacements for bone tumours of the lower limb. J Bone Joint Surg Br. 1996;78:5–13. - PubMed
-
- Myers GJ, Abudu AT, Carter SR, Tillman RM, Grimer RJ. Endoprosthetic replacement of the distal femur for bone tumours: long-term results. J Bone Joint Surg Br. 2007;89:521–526. - PubMed
LinkOut - more resources
Full Text Sources

