Validation of an omega-3 substrate challenge absorption test as an indicator of global fat lipolysis
- PMID: 37155649
- PMCID: PMC10166528
- DOI: 10.1371/journal.pone.0284651
Validation of an omega-3 substrate challenge absorption test as an indicator of global fat lipolysis
Abstract
Introduction: The coefficient of fat absorption (CFA) quantifies fat that remains in stool after digestion and is not a direct measure of lipolysis. CFA has been used to assess treatment of pancreatic insufficiency but does not correlate with pancreatic enzyme replacement therapy dose. We explored use of an omega-3 substrate absorption challenge test as a sensitive test of lipolysis and absorption.
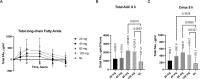
Methods: We studied a novel microbially-derived lipase (SNSP003) employing an established surgical model commonly used to study the uptake of macronutrients, the exocrine pancreatic insufficient pig. Pigs were fed a high-fat diet and given a standardized omega-3 substrate challenge to test the effect of lipolysis on its absorption. Blood was drawn at 0, 1, 2, 4, 6, 8, 12, and 24 hours following the substrate challenge and was analyzed for omega-3 and total fat levels (c14:c24). SNSP003 was also compard to porcine pancrelipase.
Results: The absorption of omega-3 fats was significantly increased following administration of 40, 80 and 120 mg SNSP003 lipase by 51% (p = 0.02), 89%, (p = 0.001) and 64% (p = 0.01), respectively, compared to that observed when no lipase was administered to the pigs, with Tmax at 4 hours. The two highest SNSP003 doses were compared to porcine pancrelipase and no significant differences were observed. Both doses increased plasma total fatty acids (141% for the 80 mg dose (p = 0.001) and 133% for the 120 mg dose (p = 0.006), compared to no lipase) and no significant differences were observed between the SNSP003 lipase doses and porcine pancrelipase.
Conclusion: The omega-3 substrate absorption challenge test differentiates among different doses of a novel microbially-derived lipase and correlates with global fat lipolysis and absorption in exocrine pancreatic insufficient pigs. No significant differences were observed between the two highest novel lipase doses and porcine pancrelipase. Studies in humans should be designed to support the evidence presented here that suggests the omega-3 substrate absorption challenge test has advantages over the coefficient of fat absorption test to study lipase activity.
Copyright: © 2023 Freedman et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The research was sponsored by Synspira Therapeutics, Inc. SF, MS and DB are members of the Synspira Therapeutics Scientific Advisory Board and are reimbursed for their efforts. DB is a member of the Synspira Therapeutics Board of Directors and receives compensation in stock. RG is the President and CEO of Synspira Therapeutics, Inc. There are no patents, products in development or marketed products associated with this research to declare. The product tested (SNSP003) is about to enter human clinical trials and thus is in development, and patents have been filed on SNSP003. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
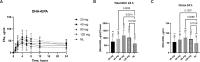


References
-
- Transcript for the January 12, 2011 Meeting of the Gastrointestinal Drugs Advisory Committee,. 2011; Available from: https://wayback.archive-it.org/7993/20170404152520/https://www.fda.gov/d....
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

