MyosinA is a druggable target in the widespread protozoan parasite Toxoplasma gondii
- PMID: 37155705
- PMCID: PMC10185354
- DOI: 10.1371/journal.pbio.3002110
MyosinA is a druggable target in the widespread protozoan parasite Toxoplasma gondii
Abstract
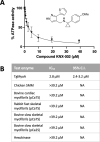
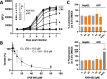
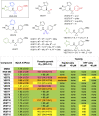
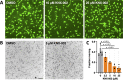
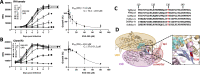
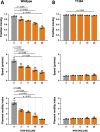
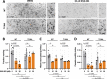
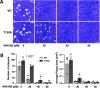
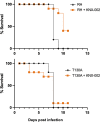
Toxoplasma gondii is a widespread apicomplexan parasite that can cause severe disease in its human hosts. The ability of T. gondii and other apicomplexan parasites to invade into, egress from, and move between cells of the hosts they infect is critical to parasite virulence and disease progression. An unusual and highly conserved parasite myosin motor (TgMyoA) plays a central role in T. gondii motility. The goal of this work was to determine whether the parasite's motility and lytic cycle can be disrupted through pharmacological inhibition of TgMyoA, as an approach to altering disease progression in vivo. To this end, we first sought to identify inhibitors of TgMyoA by screening a collection of 50,000 structurally diverse small molecules for inhibitors of the recombinant motor's actin-activated ATPase activity. The top hit to emerge from the screen, KNX-002, inhibited TgMyoA with little to no effect on any of the vertebrate myosins tested. KNX-002 was also active against parasites, inhibiting parasite motility and growth in culture in a dose-dependent manner. We used chemical mutagenesis, selection in KNX-002, and targeted sequencing to identify a mutation in TgMyoA (T130A) that renders the recombinant motor less sensitive to compound. Compared to wild-type parasites, parasites expressing the T130A mutation showed reduced sensitivity to KNX-002 in motility and growth assays, confirming TgMyoA as a biologically relevant target of KNX-002. Finally, we present evidence that KNX-002 can slow disease progression in mice infected with wild-type parasites, but not parasites expressing the resistance-conferring TgMyoA T130A mutation. Taken together, these data demonstrate the specificity of KNX-002 for TgMyoA, both in vitro and in vivo, and validate TgMyoA as a druggable target in infections with T. gondii. Since TgMyoA is essential for virulence, conserved in apicomplexan parasites, and distinctly different from the myosins found in humans, pharmacological inhibition of MyoA offers a promising new approach to treating the devastating diseases caused by T. gondii and other apicomplexan parasites.
Copyright: © 2023 Kelsen et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Comment in
-
Stopping a parasite in its tracks.Nat Rev Microbiol. 2023 Jul;21(7):413. doi: 10.1038/s41579-023-00915-0. Nat Rev Microbiol. 2023. PMID: 37208460 No abstract available.
Similar articles
-
Blocking Palmitoylation of Toxoplasma gondii Myosin Light Chain 1 Disrupts Glideosome Composition but Has Little Impact on Parasite Motility.mSphere. 2021 May 19;6(3):e00823-20. doi: 10.1128/mSphere.00823-20. mSphere. 2021. PMID: 34011689 Free PMC article.
-
Calcium-dependent phosphorylation alters class XIVa myosin function in the protozoan parasite Toxoplasma gondii.Mol Biol Cell. 2014 Sep 1;25(17):2579-91. doi: 10.1091/mbc.E13-11-0648. Epub 2014 Jul 2. Mol Biol Cell. 2014. PMID: 24989796 Free PMC article.
-
Functional and biophysical analyses of the class XIV Toxoplasma gondii myosin D.J Muscle Res Cell Motil. 2006;27(2):139-51. doi: 10.1007/s10974-005-9046-1. Epub 2006 Feb 10. J Muscle Res Cell Motil. 2006. PMID: 16470333
-
How does Toxoplama gondii invade host cells?J Vet Med Sci. 2018 Nov 23;80(11):1702-1706. doi: 10.1292/jvms.18-0344. Epub 2018 Oct 4. J Vet Med Sci. 2018. PMID: 30282883 Free PMC article. Review.
-
Toxoplasma gondii: perfecting an intracellular life style.Traffic. 2003 Sep;4(9):581-6. doi: 10.1034/j.1600-0854.2003.00117.x. Traffic. 2003. PMID: 12911812 Review.
Cited by
-
Stopping a parasite in its tracks.Nat Rev Microbiol. 2023 Jul;21(7):413. doi: 10.1038/s41579-023-00915-0. Nat Rev Microbiol. 2023. PMID: 37208460 No abstract available.
-
Myosin A and F-Actin play a critical role in mitochondrial dynamics and inheritance in Toxoplasma gondii.PLoS Pathog. 2024 Oct 7;20(10):e1012127. doi: 10.1371/journal.ppat.1012127. eCollection 2024 Oct. PLoS Pathog. 2024. PMID: 39374269 Free PMC article.
-
New advances in immune mechanism and treatment during ocular toxoplasmosis.Front Immunol. 2024 May 10;15:1403025. doi: 10.3389/fimmu.2024.1403025. eCollection 2024. Front Immunol. 2024. PMID: 38799473 Free PMC article. Review.
-
Myosin A and F-Actin play a critical role in mitochondrial dynamics and inheritance in Toxoplasma gondii.bioRxiv [Preprint]. 2024 Mar 18:2024.03.18.585462. doi: 10.1101/2024.03.18.585462. bioRxiv. 2024. Update in: PLoS Pathog. 2024 Oct 7;20(10):e1012127. doi: 10.1371/journal.ppat.1012127. PMID: 38562694 Free PMC article. Updated. Preprint.
-
Motility-dependent processes in Toxoplasma gondii tachyzoites and bradyzoites: same same but different.bioRxiv [Preprint]. 2024 Sep 28:2024.09.28.615543. doi: 10.1101/2024.09.28.615543. bioRxiv. 2024. Update in: mSphere. 2025 Mar 25;10(3):e0085524. doi: 10.1128/msphere.00855-24. PMID: 39386639 Free PMC article. Updated. Preprint.
References
-
- El Bissati K, Levigne P, Lykins J, Adlaoui EB, Barkat A, Berraho A, et al.. Global initiative for congenital toxoplasmosis: an observational and international comparative clinical analysis. Emerg Microbes Infect. 2018;7(1):165. Epub 2018/09/29. doi: 10.1038/s41426-018-0164-4 ; PubMed Central PMCID: PMC6160433. - DOI - PMC - PubMed
-
- Vidal JE, Hernandez AV, de Oliveira AC, Dauar RF, Barbosa SP Jr., Focaccia R. Cerebral toxoplasmosis in HIV-positive patients in Brazil: clinical features and predictors of treatment response in the HAART era. AIDS Patient Care STDS. 2005;19(10):626–34. Epub 2005/10/20. doi: 10.1089/apc.2005.19.626 . - DOI - PubMed
-
- de Oliveira GB, da Silva MA, Wanderley LB, da Cunha CC, Ferreira EC, de Medeiros ZM, et al.. Cerebral toxoplasmosis in patients with acquired immune deficiency syndrome in the neurological emergency department of a tertiary hospital. Clin Neurol Neurosurg. 2016;150:23–26. doi: 10.1016/j.clineuro.2016.08.014 . - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

