Synthesis, Computational Insights, and Evaluation of Novel Sigma Receptors Ligands
- PMID: 37155827
- PMCID: PMC10197133
- DOI: 10.1021/acschemneuro.3c00074
Synthesis, Computational Insights, and Evaluation of Novel Sigma Receptors Ligands
Abstract
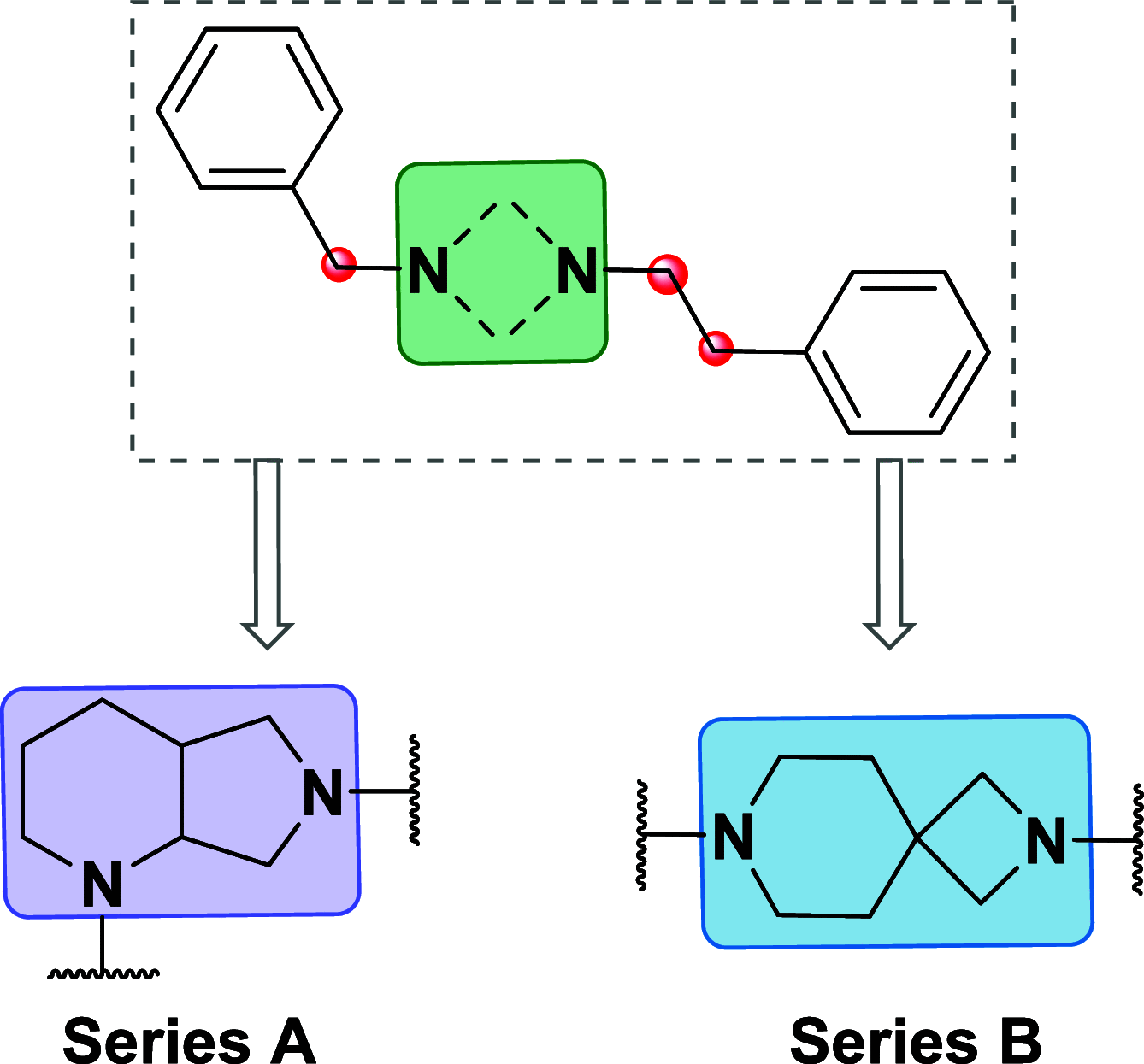
The development of diazabicyclo[4.3.0]nonane and 2,7-diazaspiro[3.5]nonane derivatives as sigma receptors (SRs) ligands is reported. The compounds were evaluated in S1R and S2R binding assays, and modeling studies were carried out to analyze the binding mode. The most notable compounds, 4b (AD186, KiS1R = 2.7 nM, KiS2R = 27 nM), 5b (AB21, KiS1R = 13 nM, KiS2R = 102 nM), and 8f (AB10, KiS1R = 10 nM, KiS2R = 165 nM), have been screened for analgesic effects in vivo, and their functional profile was determined through in vivo and in vitro models. Compounds 5b and 8f reached the maximum antiallodynic effect at 20 mg/kg. The selective S1R agonist PRE-084 completely reversed their action, indicating that the effects are entirely dependent on the S1R antagonism. Conversely, compound 4b sharing the 2,7-diazaspiro[3.5]nonane core as 5b was completely devoid of antiallodynic effect. Interestingly, compound 4b fully reversed the antiallodynic effect of BD-1063, indicating that 4b induces an S1R agonistic in vivo effect. The functional profiles were confirmed by the phenytoin assay. Our study might establish the importance of 2,7-diazaspiro[3.5]nonane core for the development of S1R compounds with specific agonist or antagonist profile and the role of the diazabicyclo[4.3.0]nonane in the development of novel SR ligands.
Keywords: S1R agonist; S1R antagonist; SR ligands; drug discovery; mechanical hypersensitivity; molecular modeling; sigma receptors.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Walker J. M.; Bowen W. D.; Walker F. O.; Matsumoto R. R.; De Costa B.; Rice K. C. Sigma receptors: biology and function. Pharmacol. Rev. 1990, 42, 355–402. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

