Circular RNA vaccine induces potent T cell responses
- PMID: 37155869
- PMCID: PMC10193964
- DOI: 10.1073/pnas.2302191120
Circular RNA vaccine induces potent T cell responses
Abstract
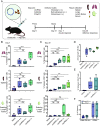
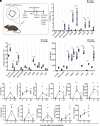
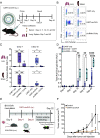
Circular RNAs (circRNAs) are a class of RNAs commonly found across eukaryotes and viruses, characterized by their resistance to exonuclease-mediated degradation. Their superior stability compared to linear RNAs, combined with previous work showing that engineered circRNAs serve as efficient protein translation templates, make circRNA a promising candidate for RNA medicine. Here, we systematically examine the adjuvant activity, route of administration, and antigen-specific immunity of circRNA vaccination in mice. Potent circRNA adjuvant activity is associated with RNA uptake and activation of myeloid cells in the draining lymph nodes and transient cytokine release. Immunization of mice with engineered circRNA encoding a protein antigen delivered by a charge-altering releasable transporter induced innate activation of dendritic cells, robust antigen-specific CD8 T cell responses in lymph nodes and tissues, and strong antitumor efficacy as a therapeutic cancer vaccine. These results highlight the potential utility of circRNA vaccines for stimulating potent innate and T cell responses in tissues.
Keywords: CD8 T cells; circular RNA; vaccine.
Conflict of interest statement
H.Y.C. is a co-founder of Accent Therapeutics, Boundless Bio, Cartography Biosciences, Orbital Therapeutics and an advisor of 10x Genomics, Arsenal Biosciences, Chroma Medicine, and Spring Discovery. P.A.W. is a co-founder of BryoLogyx and N1 Life and an advisor to BryoLogyx, N1 Life, Synaptogenix, Cytokinetics, Evonik, SuperTrans Medical, Ativo, and Vault Pharma. B.P. serves on the External Immunology Network of GSK and on the scientific advisory board of Medicago, Sanofi, EdJen, and Boehringer-Ingelheim. Stanford University has filed a patent on circular RNA technology on which L.A., B.P., and H.Y.C. are named as inventors. The remaining authors declare no competing interests.
Figures

References
-
- Kristensen L. S., et al. , The biogenesis, biology and characterization of circular RNAs. Nat. Rev. Genet. 20, 675–691 (2019). - PubMed
-
- Memczak S., et al. , Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 495, 333–338 (2013). - PubMed
-
- Hansen T. B., et al. , Natural RNA circles function as efficient microRNA sponges. Nature 495, 384–388 (2013). - PubMed
-
- Ashwal-Fluss R., et al. , circRNA biogenesis competes with pre-mRNA splicing. Mol. Cell 56, 55–66 (2014). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

