Association of Pregnancy and HIV Status With Molecular-Bacterial Vaginosis in Indian Women
- PMID: 37155962
- PMCID: PMC10524256
- DOI: 10.1097/QAI.0000000000003215
Association of Pregnancy and HIV Status With Molecular-Bacterial Vaginosis in Indian Women
Abstract
Background: Bacterial vaginosis (BV) is a highly prevalent disorder of the cervicovaginal microbiota. Molecular-BV may put women at increased risk for adverse reproductive and obstetric outcomes. We investigated the association of HIV and pregnancy on the vaginal microbiota and associations with molecular-BV in women of reproductive age from Pune, India.
Setting: We studied vaginal samples from N = 170 women, including N = 44 nonpregnant HIV seronegative, N = 56 pregnant seronegative, N = 47 nonpregnant women with HIV (WWH), and N = 23 pregnant WWH, and collected data on clinical, behavioral, and demographic factors.
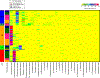
Methods: We used 16S rRNA gene amplicon sequencing to characterize the composition of the vaginal microbiota. We classified the vaginal microbiota of these women into community state types based on bacterial composition and relative abundance and further categorized them into molecular-BV versus Lactobacillus -dominated states. To determine associations between pregnancy and HIV status with outcome of molecular-BV, logistic regression models were used.
Results: There was a high prevalence of molecular-BV (30%) in this cohort. We found that pregnancy was associated with decreased odds of molecular-BV (adjusted OR = 0.35, 95% CI: 0.14 to 0.87), while HIV was associated with increased odds of molecular-BV (adjusted OR = 2.76, 95% CI: 1.33 to 5.73), even when controlling for multiple relevant factors such as age, number of sexual partners, condom use, and douching.
Conclusion: Larger and longitudinal studies are needed to further characterize molecular-BV and the vaginal microbiota in pregnant women and WWH and relate these factors to infectious, reproductive, and obstetric outcomes. In the long term, these studies may lead to novel microbiota-based therapeutics to improve women's reproductive and obstetric health.
Copyright © 2023 Wolters Kluwer Health, Inc. All rights reserved.
Conflict of interest statement
JR is co-founder of LUCA Biologics, a biotechnology company focusing on translating microbiome research into live biotherapeutics drugs for women’s health. ST has been a consultant for Biofire Diagnostics, Roche Molecular Diagnostics and LUCA Biologics, receives royalties from UPTODATE and has received speaker honoraria from Roche Molecular Diagnostics and Medscape/WebMD. For the remaining authors none were declared.
Research reported in this publication was supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under award number R00HD089753 to RS, R01HD081929 to AG, as well as NIAID (K23AI129854 to JSM). Additional support for this work was the NIH-funded Johns Hopkins Baltimore-Washington-India Clinical Trials Unit for NIAID Networks (UM1AI069465 to AG). JR, MSH and JBH were supported by the National Institute of Nursing Research, of the National Institute of Health under award number R01NR015495. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH. The authors also acknowledge in-kind support from Persistent Systems and SpeeDx.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

