Posttransplant cyclophosphamide vs tacrolimus-based GVHD prophylaxis: lower incidence of relapse and chronic GVHD
- PMID: 37156098
- PMCID: PMC10405198
- DOI: 10.1182/bloodadvances.2023009791
Posttransplant cyclophosphamide vs tacrolimus-based GVHD prophylaxis: lower incidence of relapse and chronic GVHD
Abstract
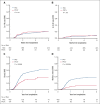
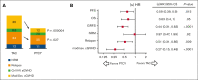
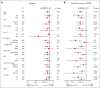
The ability of posttransplant cyclophosphamide (PTCY) to facilitate haploidentical transplantation has spurred interest in whether PTCY can improve clinical outcomes in patients with HLA-matched unrelated donors undergoing peripheral blood stem cell transplantation (PBSCT). We investigated our institutional experience using PTCY-based graft-versus-host disease (GVHD) prophylaxis compared with conventional tacrolimus-based regimens. We compared overall survival, progression-free survival (PFS), relapse, nonrelapse mortality, and acute and chronic GVHD in 107 adult patients receiving a PTCY-based regimen vs 463 patients receiving tacrolimus-based regimens for GVHD prophylaxis. The 2 cohorts were well balanced for baseline characteristics except that more patients in the PTCY cohort having received 7-of-8-matched PBSCT. There was no difference in acute GVHD. All-grade chronic GVHD and moderate-to-severe chronic GVHD were substantially reduced in patients receiving PTCY compared with in those receiving tacrolimus-based regimens (2-year moderate-to-severe chronic GVHD: 12% vs 36%; P < .0001). Recipients of PTCY-based regimens also had a lower incidence of relapse compared with recipients of tacrolimus-based regimens (25% vs 34% at 2-years; P = .027), primarily in patients who received reduced intensity conditioning. This led to improved PFS in the PTCY cohort (64% vs 54% at 2 years; P = .02). In multivariable analysis, the hazard ratio was 0.59 (P = .015) for PFS and the subdistribution hazard ratio was 0.27 (P < .0001) for moderate-to-severe chronic GVHD and 0.59 (P = .015) for relapse. Our results suggest that PTCY prophylaxis is associated with lower rates of relapse and chronic GVHD in patients who receive HLA-matched unrelated donor PBSCT.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: J.R. received research funding from Equillium, Kite Pharma, Novartis, and Oncternal, and served on advisory boards for Akron, Avrobio, Clade, Erbi, Garuda, LifeVault, Novartis, Smart Immune, Talaris, and TScan. C.J.W. holds equity in BioNTech, and receives research funding from Pharmacyclics. J.K. reports research support from Amgen, Equillium, Bristol Myers Squibb, Miltenyi Biotec, Regeneron, and Clinigen; consulting income from Amgen, Equillium, and Moderna Therapeutics; and is a scientific advisory board member for Cugene and Therakos. S.N. reports ad hoc advisory board representation for Kite/Gilead, GlaxoSmithKline, Iovance, A2 Bio, and Sobi. R.J.S. serves on the board of directors for Be The Match/National Marrow Donor Program; provided consulting for Vor Biopharma, Neovii, CSL Behring, Bluesphere Bio, Cugene, Jasper, and Smart Immune; and is on the data safety monitoring board for Juno Therapeutics. H.T.K. provided consulting for Neovii. The remaining authors declare no competing financial interests.
Figures



References
-
- Ahlmann M, Hempel G. The effect of cyclophosphamide on the immune system: implications for clinical cancer therapy. Cancer Chemother Pharmacol. 2016;78(4):661–671. - PubMed
-
- Bashey A, Zhang X, Jackson K, et al. Comparison of outcomes of hematopoietic cell transplants from T-replete haploidentical donors using post-transplantation cyclophosphamide with 10 of 10 HLA-A, -B, -C, -DRB1, and -DQB1 allele-matched unrelated donors and HLA-identical sibling donors: a multivariable analysis including disease risk index. Biol Blood Marrow Transplant. 2016;22(1):125–133. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

