Identification of novel coenzyme Q10 biosynthetic proteins Coq11 and Coq12 in Schizosaccharomyces pombe
- PMID: 37156397
- PMCID: PMC10279924
- DOI: 10.1016/j.jbc.2023.104797
Identification of novel coenzyme Q10 biosynthetic proteins Coq11 and Coq12 in Schizosaccharomyces pombe
Abstract
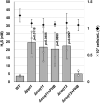
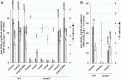
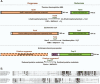
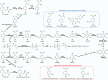
Coenzyme Q (CoQ) is an essential component of the electron transport system in aerobic organisms. CoQ10 has ten isoprene units in its quinone structure and is especially valuable as a food supplement. However, the CoQ biosynthetic pathway has not been fully elucidated, including synthesis of the p-hydroxybenzoic acid (PHB) precursor to form a quinone backbone. To identify the novel components of CoQ10 synthesis, we investigated CoQ10 production in 400 Schizosaccharomyces pombe gene-deleted strains in which individual mitochondrial proteins were lost. We found that deletion of coq11 (an S. cerevisiae COQ11 homolog) and a novel gene designated coq12 lowered CoQ levels to ∼4% of that of the WT strain. Addition of PHB or p-hydroxybenzaldehyde restored the CoQ content and growth and lowered hydrogen sulfide production of the Δcoq12 strain, but these compounds did not affect the Δcoq11 strain. The primary structure of Coq12 has a flavin reductase motif coupled with an NAD+ reductase domain. We determined that purified Coq12 protein from S. pombe displayed NAD+ reductase activity when incubated with ethanol-extracted substrate of S. pombe. Because purified Coq12 from Escherichia coli did not exhibit reductase activity under the same conditions, an extra protein is thought to be necessary for its activity. Analysis of Coq12-interacting proteins by LC-MS/MS revealed interactions with other Coq proteins, suggesting formation of a complex. Thus, our analysis indicates that Coq12 is required for PHB synthesis, and it has diverged among species.
Keywords: Coq11; Coq12; Schizosaccharomyces pombe; biosynthetic proteins; coenzyme Q(10); oxidoreductase; p-hydroxybenzoic acid.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures















References
-
- Crane F.L. The evolution of coenzyme Q. Biofactors. 2008;32:5–11. - PubMed
-
- Bentinger M., Tekle M., Dallner G. Coenzyme Q--biosynthesis and functions. Biochem. Biophys. Res. Commun. 2010;396:74–79. - PubMed
-
- Kawamukai M. Biosynthesis and applications of prenylquinones. Biosci. Biotechnol. Biochem. 2018;82:963–977. - PubMed
-
- Kawamukai M. Biosynthesis, bioproduction and novel roles of ubiquinone. J. Biosci. Bioeng. 2002;94:511–517. - PubMed
-
- Kawamukai M. Biosynthesis and bioproduction of coenzyme Q10 by yeasts and other organisms. Biotechnol. Appl. Biochem. 2009;53:217–226. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

