Self-Regulated and Bidirectional Communication in Synthetic Cell Communities
- PMID: 37156507
- PMCID: PMC10210537
- DOI: 10.1021/acsnano.2c09908
Self-Regulated and Bidirectional Communication in Synthetic Cell Communities
Abstract
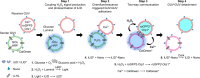
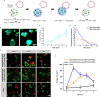
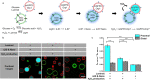
Cell-to-cell communication is not limited to a sender releasing a signaling molecule and a receiver perceiving it but is often self-regulated and bidirectional. Yet, in communities of synthetic cells, such features that render communication efficient and adaptive are missing. Here, we report the design and implementation of adaptive two-way signaling with lipid-vesicle-based synthetic cells. The first layer of self-regulation derives from coupling the temporal dynamics of the signal, H2O2, production in the sender to adhesions between sender and receiver cells. This way the receiver stays within the signaling range for the duration sender produces the signal and detaches once the signal fades. Specifically, H2O2 acts as both a forward signal and a regulator of the adhesions by activating photoswitchable proteins at the surface for the duration of the chemiluminescence. The second layer of self-regulation arises when the adhesions render the receiver permeable and trigger the release of a backward signal, resulting in bidirectional exchange. These design rules provide a concept for engineering multicellular systems with adaptive communication.
Keywords: adaptive; bidirectional communication; chemiluminescence; self-regulation; synthetic cells.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

