The incidence and prevalence of IgA nephropathy in Europe
- PMID: 37156519
- PMCID: PMC10539204
- DOI: 10.1093/ndt/gfad082
The incidence and prevalence of IgA nephropathy in Europe
Abstract
Background: This study aimed to determine the incidence and prevalence of immunoglobulin A nephropathy (IgAN) in Europe based on high-quality data from national registries.
Methods: IgAN incidences were obtained from a literature review of European studies of national kidney biopsy registry data in which IgAN diagnosis was biopsy-verified using contemporary techniques. Studies were eligible for the main analysis if published from 1990 to 2020. IgAN point prevalence was defined as the annual IgAN incidence multiplied by the estimated duration of disease. Incidence and prevalence estimates were made for three pooled populations: (i) patients of all ages; (ii) pediatric patients; and (iii) elderly patients.
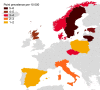
Results: Across 10 European countries, the estimated annual IgAN incidence was 0.76 per 100 000 in patients of all ages. The corresponding pooled IgAN point prevalence was 2.53 per 10 000 (95% confidence interval: 2.51-2.55), ranging from 1.14 per 10 000 in Spain to 5.98 per 10 000 in Lithuania. Applied to 2021 population estimates, the number of expected prevalent IgAN cases was 47 027 across all 10 countries and ranged from 577 in Estonia to 16 645 in Italy. Among pediatric patients, IgAN incidence was 0.20 per 100 000 children and IgAN point prevalence was 0.12 per 10 000 children. Among elderly patients, IgAN incidence was 0.30 per 100 000 and IgAN point prevalence was 0.36 per 10 000.
Conclusions: Based on high-quality data from European national registries, IgAN point prevalence was estimated at 2.53 per 10 000 in patients of all ages. Prevalence was considerably lower in pediatric and elderly populations.
Keywords: IgA; glomerulonephritis; immunoglobulin A nephropathy; incidence; prevalence; registries.
© The Author(s) 2023. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
C.J.W. has served as an epidemiologic consultant to Otsuka/Visterra, Janssen Pharmaceuticals, and Healx. R.C. has been a consultant or advisory board member for Amgen, Argenx, Calliditas Therapeutics, Menarini, Novartis, Otsuka/Visterra, Purespring, Reata Pharmaceuticals, Stadapharm, and Travere Therapeutics. F.S. has received consultancy fees from Alexion, Otsuka, and Novartis. M.M.W. declares no conflict of interest. M.M. is a full-time employee of Visterra, Inc. M.J.S. was a full-time employee of Visterra, Inc. at the time of this work, and is currently a full-time employee of Otsuka Pharmaceutical Development & Commercialization Inc.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous