Quality of life between home-based and outpatient pulmonary rehabilitation in patients after surgical resection for lung cancer: protocol for a prospective, single-blind, randomised controlled trial
- PMID: 37156593
- PMCID: PMC10174035
- DOI: 10.1136/bmjopen-2022-067845
Quality of life between home-based and outpatient pulmonary rehabilitation in patients after surgical resection for lung cancer: protocol for a prospective, single-blind, randomised controlled trial
Abstract
Introduction: Lung cancer remains a highly fatal disease. Surgical resection has been proven to be the most effective treatment for early-stage lung cancer. The conventional hospital-based pulmonary rehabilitation (PR) is shown to reduce symptoms, improve exercise capacity and impact the quality of life (QoL) for lung cancer patients. To date, scientific evidence on the effectiveness of home-based PR for patients with lung cancer following surgery is scarce. We aim to explore if home-based PR is non-inferior to outpatient PR for patients with lung cancer following surgical resection.
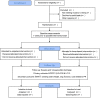
Methods and analysis: This study is a two-arm, parallel-group, assessor-blind, single-centre, randomised controlled trial. Participants will be recruited from West China Hospital, Sichuan University and randomly allocated to either an outpatient group or a home-based group at a ratio of 1:1. The PR programme involves self-management and exercises. The exercise includes warm-up (10 min), aerobic training (20 min), resistance training (15 min) and cool-down (10 min), lasting 4 weeks, with two sessions per week either at home or in the outpatient setting. The intensity will be adjusted according to the modified Borg rating of perceived exertion and heart rate before and after each exercise session. The primary outcome is QoL measured by EORTC QLQ-C30 & LC 13 after an intervention. Secondary outcomes include physical fitness measured by a 6 min walk test and stair-climbing test and symptom severity measured by patient-reported questionnaires and pulmonary function. The main hypothesis is that home-based PR is non-inferior to outpatient PR for patients with lung cancer following surgical resection.
Ethics and dissemination: The trial has been approved by the Ethical Committee of West China Hospital and is also registered with the Chinese Clinical Trial Registry. The results of this study will be disseminated through peer-reviewed publications and presentations at national and international conferences.
Trial registration number: ChiCTR2100053714.
Keywords: Rehabilitation medicine; Respiratory tract tumours; THORACIC MEDICINE.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Minimal versus specialist equipment in the delivery of pulmonary rehabilitation: protocol for a non-inferiority randomised controlled trial.BMJ Open. 2021 Oct 18;11(10):e047524. doi: 10.1136/bmjopen-2020-047524. BMJ Open. 2021. PMID: 34663653 Free PMC article.
-
Effect of an intensive 3-week preoperative home rehabilitation programme in patients with chronic obstructive pulmonary disease eligible for lung cancer surgery: a multicentre randomised controlled trial.BMJ Open. 2017 Nov 12;7(11):e017307. doi: 10.1136/bmjopen-2017-017307. BMJ Open. 2017. PMID: 29133320 Free PMC article. Clinical Trial.
-
Effects of home-based cardiac exercise rehabilitation with remote electrocardiogram monitoring in patients with chronic heart failure: a study protocol for a randomised controlled trial.BMJ Open. 2019 Mar 5;9(3):e023923. doi: 10.1136/bmjopen-2018-023923. BMJ Open. 2019. PMID: 30842109 Free PMC article.
-
Exercise training undertaken by people within 12 months of lung resection for non-small cell lung cancer.Cochrane Database Syst Rev. 2019 Jun 17;6(6):CD009955. doi: 10.1002/14651858.CD009955.pub3. Cochrane Database Syst Rev. 2019. PMID: 31204439 Free PMC article.
-
Effects of therapeutic exercises in patients with lung cancer. A scoping review.J Bodyw Mov Ther. 2022 Jul;31:22-29. doi: 10.1016/j.jbmt.2022.03.003. Epub 2022 Mar 17. J Bodyw Mov Ther. 2022. PMID: 35710217
Cited by
-
[Chinese Expert Consensus on Day Surgery Management of Lung Cancer (2024 Edition)].Zhongguo Fei Ai Za Zhi. 2024 Jun 20;27(6):405-414. doi: 10.3779/j.issn.1009-3419.2024.102.24. Zhongguo Fei Ai Za Zhi. 2024. PMID: 39026491 Free PMC article. Chinese.
-
Research designs for cardiothoracic surgeons: part 1 - a primer for evidence-based practice.Indian J Thorac Cardiovasc Surg. 2024 Nov;40(6):737-751. doi: 10.1007/s12055-024-01836-0. Epub 2024 Oct 4. Indian J Thorac Cardiovasc Surg. 2024. PMID: 39416325
References
-
- Horn L, Spigel DR, Vokes EE, et al. . Nivolumab versus docetaxel in previously treated patients with advanced non–small-cell lung cancer: two-year outcomes from two randomized, open-label, phase III trials (checkmate 017 and checkmate 057). JCO 2017;35:3924–33. 10.1200/JCO.2017.74.3062 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials