Indicators of Tobacco Dependence Among Youth: Findings From Wave 1 (2013-2014) of the Population Assessment of Tobacco and Health Study
- PMID: 37156636
- PMCID: PMC10439486
- DOI: 10.1093/ntr/ntad072
Indicators of Tobacco Dependence Among Youth: Findings From Wave 1 (2013-2014) of the Population Assessment of Tobacco and Health Study
Erratum in
-
Correction to 24 papers to add an additional interest disclosure.Nicotine Tob Res. 2024 Dec 23;27(1):163. doi: 10.1093/ntr/ntae234. Nicotine Tob Res. 2024. PMID: 39405446 Free PMC article. No abstract available.
Abstract
Background: Prior work established a measure of tobacco dependence (TD) among adults that can be used to compare TD across different tobacco products. We extend this approach to develop a common, cross-product metric for TD among youth.
Methods: One thousand one hundred and forty-eight youth aged 12-17 who used a tobacco product in the past 30 days were identified from 13 651 youth respondents in Wave 1 of the Population Assessment of Tobacco and Health (PATH) Study.
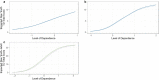
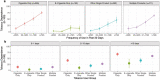
Findings: Analyses confirmed a single primary latent construct underlying responses to TD indicators for all mutually exclusive tobacco product user groups. Differential Item Functioning analyses supported the use of 8 of 10 TD indicators for comparisons across groups. With TD levels anchored at 0.0 (standard deviation [SD] = 1.0) among cigarette only (n = 265) use group, mean TD scores were more than a full SD lower for e-cigarette only (n = 150) use group (mean = -1.09; SD = 0.64). Other single product use group (cigar, hookah, pipe, or smokeless; n = 262) on average had lower TD (mean = -0.60; SD = 0.84), and the group with the use of multiple tobacco products (n = 471) experienced similar levels of TD (mean = 0.14; SD = 0.78) as the cigarette only use group. Concurrent validity was established with product use frequency among all user groups. A subset of five TD items comprised a common metric permitting comparisons between youth and adults.
Conclusion: The PATH Study Youth Wave 1 Interview provided psychometrically valid measures of TD that enable future regulatory investigations of TD across tobacco products and comparisons between youth and adult tobacco product use group.
Implications: A measure of tobacco dependence (TD) has been established previously among adults to compare TD across tobacco products. This study established the validity of a similar, cross-product measure of TD among youth. Findings suggest a single latent TD construct underlying this measure, concurrent validity of the scale with product use frequency across different types of tobacco users, and a subset of common items that can be used to compare TD between youth and adults who use tobacco.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Society for Research on Nicotine and Tobacco. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
RN receives funding from the Food and Drug Administration Center for Tobacco Products via contractual mechanisms with Westat and the National Institutes of Health. Within the past three years, he has served as a paid consultant to the Government of Canada via a contract with Industrial Economics Inc, and has received an honorarium for a virtual meeting from Pfizer Inc. RN was an unpaid grant reviewer for the Foundation for a Smoke Free World. KMC provides expert testimony on the health effects of smoking and tobacco industry tactics in lawsuits filed against the tobacco industry. He has also received payment as a consultant to Pfizer, Inc, for services on an external advisory panel to assess ways to improve smoking cessation delivery in health care settings. GTF has a Senior Investigator Award from the Ontario Institute for Cancer Research (IA-004). Over the past two years, he has received grant funding from the U.S. National Cancer Institute (5P01CA200512), Canadian Institutes of Health Research (FDN-148477), Health Research Council of New Zealand, Health Canada, Korea Health Promotion Institute, National Cancer Center Japan, Santé Publique France, Bill and Melinda Gates Foundation, British Heart Foundation, Mayo Foundation for Medical Education and Research, Cancer Research UK, and the Dutch Lung Foundation. He has been an expert witness/consultant for the governments of Uruguay and Australia defending their country’s policies/regulations in litigation, and an expert consultant for the government of Singapore regarding policies and regulations on tobacco products. He has served as a member of the Expert Group on Articles 9/10 of the WHO Framework Convention on Tobacco Control, the WHO Expert Group on COVID-19 and Tobacco Use, and Health Canada’s Scientific Advisory Board on Vaping Products. Tara Elton-Marshall acknowledges funding from Canadian Institutes of Health Research (CIHR) for the Ontario CRISM Node Team (grant #SMN-139150) and the Team Grant: Partnerships for Cannabis Policy Evaluation.
Figures
References
-
- Nonnemaker J, Mowery P, Hersey J, et al. . Measurement properties of a nicotine dependence scale for adolescents. Nicotine Tob Res. 2004;6(2):295–301. - PubMed
-
- DiFranza JR, Savageau JA, Fletcher K, et al. . Measuring the loss of autonomy over nicotine use in adolescents: the DANDY (Development and Assessment of Nicotine Dependence in Youths) study. Arch Pediatr Adolesc Med. 2002;156(4):397–403. - PubMed
-
- O’Loughlin J, DiFranza J, Tyndale RF, et al. . Nicotine-dependence symptoms are associated with smoking frequency in adolescents. Am J Prev Med. 2003;25(3):219–225. - PubMed
-
- Colby SM, Tiffany ST, Shiffman S, Niaura RS.. Are adolescent smokers dependent on nicotine? A review of the evidence. Drug Alcohol Depend. 2000;59(suppl 1):S83–S95. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

