Intrathecal trastuzumab versus alternate routes of delivery for HER2-targeted therapies in patients with HER2+ breast cancer leptomeningeal metastases
- PMID: 37156650
- PMCID: PMC10300571
- DOI: 10.1016/j.breast.2023.04.008
Intrathecal trastuzumab versus alternate routes of delivery for HER2-targeted therapies in patients with HER2+ breast cancer leptomeningeal metastases
Abstract
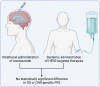
Background: Patients with HER2+ breast cancer (BC) frequently develop leptomeningeal metastases (LM). While HER2-targeted therapies have demonstrated efficacy in the neoadjuvant, adjuvant, and metastatic settings, including for parenchymal brain metastases, their efficacy for patients with LM has not been studied in a randomized controlled trial. However, several single-armed prospective studies, case series and case reports have studied oral, intravenous, or intrathecally administered HER2-targeted therapy regimens for patients with HER2+ BC LM.
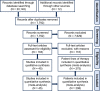
Methods: We conducted a systematic review and meta-analysis of individual patient data to evaluate the efficacy of HER2-targeted therapies in HER2+ BC LM in accordance with PRISMA guidelines. Targeted therapies evaluated were trastuzumab (intrathecal or intravenous), pertuzumab, lapatinib, neratinib, tucatinib, trastuzumab-emtansine and trastuzumab-deruxtecan. The primary endpoint was overall survival (OS), with CNS-specific progression-free survival (PFS) as a secondary endpoint.
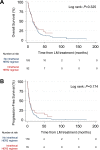
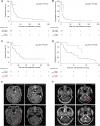
Results: 7780 abstracts were screened, identifying 45 publications with 208 patients, corresponding to 275 lines of HER2-targeted therapy for BC LM which met inclusion criteria. In univariable and multivariable analyses, we observed no significant difference in OS and CNS-specific PFS between intrathecal trastuzumab compared to oral or intravenous administration of HER2-targeted therapy. Anti-HER2 monoclonal antibody-based regimens did not demonstrate superiority over HER2 tyrosine kinase inhibitors. In a cohort of 15 patients, treatment with trastuzumab-deruxtecan was associated with prolonged OS compared to other HER2-targeted therapies and compared to trastuzumab-emtansine.
Conclusions: The results of this meta-analysis, comprising the limited data available, suggest that intrathecal administration of HER2-targeted therapy for patients with HER2+ BC LM confers no additional benefit over oral and/or IV treatment regimens. Although the number of patients receiving trastuzumab deruxtecan in this cohort is small, this novel agent offers promise for this patient population and requires further investigation in prospective studies.
Keywords: Breast cancer; Deruxtecan; Intrathecal; Leptomeningeal; Trastuzumab.
Copyright © 2023 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Efficacy of tucatinib for HER2-positive metastatic breast cancer after HER2-targeted therapy: a network meta-analysis.Future Oncol. 2021 Nov;17(33):4635-4647. doi: 10.2217/fon-2021-0742. Epub 2021 Aug 31. Future Oncol. 2021. PMID: 34463120
-
Efficacy and safety of HER2 inhibitors in combination with or without pertuzumab for HER2-positive breast cancer: a systematic review and meta-analysis.BMC Cancer. 2019 Oct 21;19(1):973. doi: 10.1186/s12885-019-6132-0. BMC Cancer. 2019. PMID: 31638935 Free PMC article.
-
A randomized, controlled phase II trial of neoadjuvant ado-trastuzumab emtansine, lapatinib, and nab-paclitaxel versus trastuzumab, pertuzumab, and paclitaxel in HER2-positive breast cancer (TEAL study).Breast Cancer Res. 2019 Sep 2;21(1):100. doi: 10.1186/s13058-019-1186-0. Breast Cancer Res. 2019. PMID: 31477168 Free PMC article. Clinical Trial.
-
Intrathecal administration of anti-HER2 treatment for the treatment of meningeal carcinomatosis in breast cancer: A metanalysis with meta-regression.Cancer Treat Rev. 2020 Aug;88:102046. doi: 10.1016/j.ctrv.2020.102046. Epub 2020 Jun 3. Cancer Treat Rev. 2020. PMID: 32599393
-
Impact of Pertuzumab and Ado-Trastuzumab Emtansine on Cumulative Avoidance of Recurrence Among Women Treated for Locally Advanced, Inflammatory, or Early-Stage Nonmetastatic HER2-Positive Breast Cancer in the United States.Adv Ther. 2023 Sep;40(9):3857-3874. doi: 10.1007/s12325-023-02554-6. Epub 2023 Jun 26. Adv Ther. 2023. PMID: 37358705 Free PMC article.
Cited by
-
Cerebral spinal fluid analyses and therapeutic implications for leptomeningeal metastatic disease.J Neurooncol. 2025 Mar;172(1):31-40. doi: 10.1007/s11060-024-04902-0. Epub 2024 Dec 20. J Neurooncol. 2025. PMID: 39704899 Review.
-
Leptomeningeal Disease: Current Approaches and Future Directions.Curr Neurol Neurosci Rep. 2025 Mar 18;25(1):25. doi: 10.1007/s11910-025-01412-y. Curr Neurol Neurosci Rep. 2025. PMID: 40100294 Free PMC article. Review.
-
Antibody-Drug Conjugates for the Treatment of Non-Small Cell Lung Cancer with Central Nervous System Metastases.Curr Oncol. 2024 Oct 18;31(10):6314-6342. doi: 10.3390/curroncol31100471. Curr Oncol. 2024. PMID: 39451775 Free PMC article. Review.
-
Clinical Activity of Mitogen-Activated Protein Kinase Inhibitors in Patients With MAP2K1 (MEK1)-Mutated Metastatic Cancers.JCO Precis Oncol. 2025 Jan;9:e2400199. doi: 10.1200/PO.24.00199. Epub 2025 Jan 27. JCO Precis Oncol. 2025. PMID: 39869838 Free PMC article.
-
Exit pathways of therapeutic antibodies from the brain and retention strategies.iScience. 2023 Oct 6;26(11):108132. doi: 10.1016/j.isci.2023.108132. eCollection 2023 Nov 17. iScience. 2023. PMID: 37915602 Free PMC article. Review.
References
-
- Le Rhun E., Taillibert S., Zairi F., et al. A retrospective case series of 103 consecutive patients with leptomeningeal metastasis and breast cancer. J Neuro Oncol. 2013;113(1):83–92. - PubMed
-
- Mills M.N., King W., Soyano A., et al. Evolving management of HER2+ breast cancer brain metastases and leptomeningeal disease. J Neuro Oncol. 2022;157(2):249–269. - PubMed
-
- Bonneau C., Paintaud G., Tredan O., et al. Phase I feasibility study for intrathecal administration of trastuzumab in patients with HER2 positive breast carcinomatous meningitis. Eur J Cancer. 2018;95:75–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

