Isatuximab, carfilzomib, and dexamethasone in patients with relapsed multiple myeloma: updated results from IKEMA, a randomized Phase 3 study
- PMID: 37156782
- PMCID: PMC10166682
- DOI: 10.1038/s41408-023-00797-8
Isatuximab, carfilzomib, and dexamethasone in patients with relapsed multiple myeloma: updated results from IKEMA, a randomized Phase 3 study
Erratum in
-
Correction: Isatuximab, carfilzomib, and dexamethasone in patients with relapsed multiple myeloma: updated results from IKEMA, a randomized Phase 3 study.Blood Cancer J. 2023 Sep 27;13(1):152. doi: 10.1038/s41408-023-00923-6. Blood Cancer J. 2023. PMID: 37752114 Free PMC article. No abstract available.
Abstract
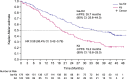
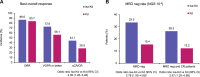
Longer-term outcomes with the anti-CD38 antibody isatuximab in combination with carfilzomib-dexamethasone (Isa-Kd) were evaluated in the randomized Phase 3 trial IKEMA (NCT03275285), in a prespecified, follow-up analysis of progression-free survival (PFS, primary study endpoint), final complete response (CR) using Hydrashift Isa immunofixation assay, minimal residual disease (MRD) negativity, and safety. Enrolled patients had relapsed/refractory multiple myeloma (1-3 prior treatment lines). Isa 10 mg/kg was administered intravenously weekly in cycle 1 then biweekly. Efficacy analyses were performed in the intent-to-treat population (Isa-Kd: n = 179, Kd: n = 123) and safety evaluated in treated patients (Isa-Kd: n = 177, Kd: n = 122). Consistent with the primary interim analysis, the addition of Isa to Kd prolonged PFS (HR 0.58, 95.4% CI: 0.42-0.79; median PFS 35.7 [95% CI: 25.8-44.0] vs 19.2 [95% CI: 15.8-25.0] months). PFS benefit was observed with Isa-Kd across subgroups, including patients with poor prognosis. The stringent CR/CR rate was 44.1% vs 28.5% (odds-ratio: 2.09, 95% CI: 1.26-3.48), the MRD negativity rate 33.5% vs 15.4% (odds-ratio: 2.78, 95% CI: 1.55-4.99) and the MRD negativity CR rate 26.3% vs 12.2%, with Isa-Kd vs Kd. The safety profile of Isa-Kd was similar to that reported in the prior interim analysis. These findings further support Isa-Kd as a standard-of-care treatment for relapsed multiple myeloma patients.Clinical trial information: ClinicalTrials.gov, NCT03275285.
© 2023. The Author(s).
Conflict of interest statement
TM: research funding (to institution) from Sanofi; participation on a steering committee for Sanofi. M-AD: participation in advisory boards for Amgen, BeiGene, Bristol Myers Squibb, Janssen, and Takeda. JM: honoraria from Amgen, Celgene, GlaxoSmithKline, Janssen, Karyopharm, Sanofi, and Takeda. KY: research funding from Bristol Myers Squibb, Janssen, and Sanofi; honoraria and travel support from Amgen, Sanofi, and Takeda; participation on an advisory board or steering committee for Janssen and Sanofi. MC: participation in speaker’s bureau for Amgen, Janssen, and Sanofi. TF: participation on a data safety monitoring board or advisory board for Amgen, Bristol Myers Squibb, Janssen, Karyopharm, Oncopeptides, Roche, and Sanofi; speakers’ bureau for Bristol Myers Squibb and Janssen. RH: honoraria and consulting/advisory role for AbbVie, Amgen, Bristol Myers Squibb, Celgene, Janssen, Novartis, PharmaMar, and Takeda; research funding (to institution) from Amgen, Bristol Myers Squibb, Celgene, Janssen, Novartis, and Takeda; participation on a data safety monitoring board or advisory board for Amgen, Bristol Myers Squibb, GSK, Janssen, Oncopeptides, Sanofi, and Takeda; support for attending meetings and/or travel from Amgen, Celgene, Janssen, and Takeda. IŠ: research funding, honoraria, and participation on a data safety monitoring board or advisory board for Amgen, Bristol Myers Squibb, Celgene, Janssen-Cilag, Novartis, PharmaMar, Sanofi, and Takeda. RB: research funding from AbbVie, Acerta Pharma, Alexion, Amgen, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, CSL Behring, Daiichi Sankyo, Janssen-Cilag, MorphoSys, Pfizer, Rigel Pharmaceuticals, Roche, Sanofi, and Takeda; honoraria from Bayer; consulting or advisory role for Janssen-Cilag and Roche; speaker’s bureau for Bayer. KK: research funding from Bristol Myers Squibb and Janssen. GM: nothing to disclose. C-KM: nothing to disclose. LP: nothing to disclose. XL: nothing to disclose. AO: honoraria from Amgen, Bristol Myers Squibb, Celgene, GlaxoSmithKline, and Sanofi; participation on a data safety monitoring board or advisory board for Amgen, Bristol Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Karyopharm, Oncopeptides, and Sanofi. YK: nothing to disclose. KS: honoraria from AbbVie, Amgen, Bristol Myers Squibb, Celgene, Janssen, Novartis, Ono Pharmaceutical, Sanofi, and Takeda. FC: employed by Ividata Life Science, contracted by Sanofi. SM: employed by Sanofi; may hold stock and/or stock options. M-LR: employed by Sanofi; may hold stock and/or stock options. PM: honoraria and consulting/advisory role for AbbVie, Amgen, Celgene, Janssen, Oncopeptides, Roche, and Sanofi.
Figures


References
-
- Dimopoulos MA, Moreau P, Palumbo A, Joshua D, Pour L, Hájek R, et al. ENDEAVOR Investigators. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17:27–38. doi: 10.1016/S1470-2045(15)00464-7. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

