Detection, quantification, and characterization of polystyrene microplastics and adsorbed bisphenol A contaminant using electroanalytical techniques
- PMID: 37156867
- PMCID: PMC10167125
- DOI: 10.1007/s00604-023-05780-5
Detection, quantification, and characterization of polystyrene microplastics and adsorbed bisphenol A contaminant using electroanalytical techniques
Abstract
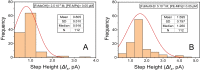
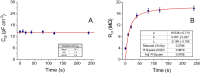
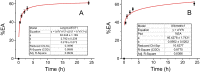
The potential applications of electroanalytical techniques for the quantification and size characterization of nonelectroactive polystyrene microplastics is reported, in addition to characterizing the kinetics of adsorption of bisphenol A on these polystyrene microparticles. The individual adsorption events of very diluted polystyrene microparticles dispersions on glassy-carbon microelectrodes produce the blocking of the charge transfer of a mediator (ferrocene-methanol) thus decreasing the current of the recorded chronoamperogram in a stepwise manner. The magnitude of the current steps are in the order of pA values and can be related to the diameter of the plastic microparticles in the size range 0.1 to 10 µm. The frequency of the current steps in the domain time used (120 s) allows to quantify the number concentration of these microparticles in the range 0.005 to 0.500 pM. Electrochemical impedance spectroscopy confirms the adsorption of the polystyrene microplastics on carbon microelectrodes (and to a lesser extent on platinum microelectrodes) under the same experimental conditions as above. On the other hand, the adsorbed microplastics become concentrators of other pollutants found in the environment. The sensitive differential-pulse voltammetry determination of bisphenol A (linear range 0.80-15.00 µM; detection limit 0.24 µM) was used together with a simple separation procedure for studying the adsorption of bisphenol A on polystyrene microparticles. The adsorption capacity (mg of bisphenol A retained per g of the polystyrene microplastics) decreased from approximately 5.7 to 0.8 mg g-1 with increasing dosages of polystyrene microparticles from 0.2 to 1.6 g l-1. The adsorption isotherms were modeled resulting in a monolayer of bisphenol A adsorbed on the microplastics (i.e., best fitted to a Langmuir model).
Keywords: Adsorptive enrichment; Blocking nanoimpact electrochemistry; Chronoamperometry; Electrochemical impedance spectroscopy; Polystyrene microparticles.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Qiu F, Peng M, Wei Z, Wang X, Yang J. Preparation of polyethersulfone/sulfonated polyethersulfonephenylethane microspheres and its application for the adsorption of bisphenol A. J Appl Polym Sci. 2016;133:43066–43074. doi: 10.1002/app.43066. - DOI
-
- Fu SS, Zhu Y, Zhang Y, Zhang MJ, Zhang YY, Qiao L, Yin N, Song KX, Liu MS, Wang DB. Recent advances in carbon nanomaterials-based electrochemical sensors for phenolic compounds detection. Microchem J. 2021;171:106776–106799. doi: 10.1016/j.microc.2021.106776. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
