Characterization of high-yield Bacillus subtilis cysteine protease for diverse industrial applications
- PMID: 37157054
- PMCID: PMC10235272
- DOI: 10.1007/s42770-023-00992-6
Characterization of high-yield Bacillus subtilis cysteine protease for diverse industrial applications
Abstract
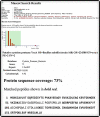
Bacterial proteases have extensive applications in various fields of industrial microbiology. In this study, protease-producing organisms were screened on skimmed milk agar media using serial dilution. Through microbial biomass production, biochemical tests, protease-specific activity, and 16 s RNA gene sequencing, the isolates were identified as Bacillus subtilis and submitted to NCBI. The strain accession numbers were designated as A1 (MT903972), A2 (MT903996), A4 (MT904091), and A5 (MT904796). The strain A4 Bacillus subtilis showed highest protease-specific activity as 76,153.84 U/mg. A4 Bacillus subtilis was unaffected by Ca2+, Cu2+, Fe2+, Hg2+, Mg2+, Na, Fe2+, and Zn2+ but was inhibited by 80% by Mn2+ (5 mM). The protease activity was inhibited by up to 30% by iodoacetamide (5 mM). These findings confirm the enzyme to be a cysteine protease which was further confirmed by MALDI-TOF. The identified protease showed 71% sequence similarity with Bacillus subtilis cysteine protease. The crude cysteine protease significantly aided in fabric stain removal when added to a generic detergent. It also aided in the recovery of silver from used X-ray films and de-hairing of goat skin hides and showed decent application in meat tenderization. Thus, the isolated cysteine protease has high potential for industrial applications.
Keywords: Cysteine protease; MALDI-TOF; Metal ions; Protease industrial applications; Protease inhibitors; Skimmed milk agar.
© 2023. The Author(s) under exclusive licence to Sociedade Brasileira de Microbiologia.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Asha B, Palaniswamy M. Optimization of alkaline protease production by Bacillus cereus FT 1 isolated from soil. J Appl Pharm Sci. 2018;8(02):119–127. doi: 10.7324/JAPS.2018.8219. - DOI
-
- Satbir S, Bijender K. Potential application spectrum of microbial proteases for clean and green industrial production. Energ. Ecol. Environ. 2017;2(6):370–386. doi: 10.1007/s40974-017-0076-5#citeas. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

