LIPUS-SCs-Exo promotes peripheral nerve regeneration in cavernous nerve crush injury-induced ED rats via PI3K/Akt/FoxO signaling pathway
- PMID: 37157936
- PMCID: PMC10580359
- DOI: 10.1111/cns.14256
LIPUS-SCs-Exo promotes peripheral nerve regeneration in cavernous nerve crush injury-induced ED rats via PI3K/Akt/FoxO signaling pathway
Abstract
Objective: Clinical treatment of erectile dysfunction (ED) caused by cavernous nerve (CN) injury during pelvic surgery is difficult. Low-intensity pulsed ultrasound (LIPUS) can be a potential strategy for neurogenic ED (NED). However, whether Schwann cells (SCs) can respond to LIPUS stimulation signals is unclear. This study aims to elucidate the signal transmission between SCs paracrine exosome (Exo) and neurons stimulated by LIPUS, as well as to analyze the role and potential mechanisms of exosomes in CN repair after injury.
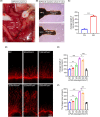
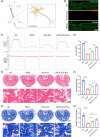
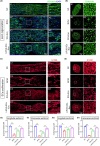
Methods: The major pelvic ganglion (MPG) neurons and MPG/CN explants were stimulated with LIPUS of different energy intensities to explore the appropriate LIPUS energy intensity. The exosomes were isolated and purified from LIPUS-stimulated SCs (LIPUS-SCs-Exo) and non-stimulated SCs (SCs-Exo). The effects of LIPUS-SCs-Exo on neurite outgrowth, erectile function, and cavernous penis histology were identified in bilateral cavernous nerve crush injury (BCNI)-induced ED rats.
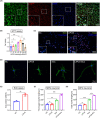
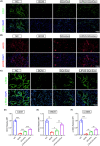
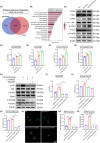
Results: LIPUS-SCs-Exo group can enhance the axon elongation of MPG/CN and MPG neurons compared to SCs-Exo group in vitro. Then, the LIPUS-SCs-Exo group showed a stronger ability to promote the injured CN regeneration and SCs proliferation compared to the SCs-Exo group in vivo. Furthermore, the LIPUS-SCs-Exo group increased the Max intracavernous pressure (ICP)/mean arterial pressure (MAP), lumen to parenchyma and smooth muscle to collagen ratios compared to the SCs-Exo group in vivo. Additionally, high-throughput sequencing combined with bioinformatics analysis revealed the differential expression of 1689 miRNAs between the SCs-Exo group and the LIPUS-SCs-Exo group. After LIPUS-SCs-Exo treatment, the phosphorylated levels of Phosphatidylinositol 3-kinase (PI3K), protein kinase B (Akt) and forkhead box O (FoxO) in MPG neurons increased significantly compared to negative control (NC) and SCs-Exo groups.
Conclusion: Our study revealed that LIPUS stimulation could regulate the gene of MPG neurons by changing miRNAs derived from SCs-Exo, then activating the PI3K-Akt-FoxO signal pathway to enhance nerve regeneration and restore erectile function. This study had important theoretical and practical significance for improving the NED treatment.
Keywords: Schwann cells; erectile dysfunction; exosomes; low-intensity pulsed ultrasound; nerve regeneration.
© 2023 The Authors. CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Shamloul R, Ghanem H. Erectile dysfunction. Lancet (London, England). 2013;381(9861):153‐165. - PubMed
-
- Corona G, Rastrelli G, Morgentaler A, Sforza A, Mannucci E, Maggi M. Meta‐analysis of results of testosterone therapy on sexual function based on international index of erectile function scores. Eur Urol. 2017;72(6):1000‐1011. - PubMed
-
- Kendirci M, Tanriverdi O, Trost L, Hellstrom WJG. Management of sildenafil treatment failures. Curr Opin Urol. 2006;16(6):449‐459. - PubMed
-
- Del Popolo G, Cito G, Gemma L, et al. Neurogenic sexual dysfunction treatment: a systematic review. Eur Urol Focus. 2020;6(5):868‐876. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

