Biological aspects to enhance fracture healing
- PMID: 37158338
- PMCID: PMC10233810
- DOI: 10.1530/EOR-23-0047
Biological aspects to enhance fracture healing
Abstract
The ability to enhance fracture healing is paramount in modern orthopaedic trauma, particularly in the management of challenging cases including peri-prosthetic fractures, non-union and acute bone loss. Materials utilised in enhancing fracture healing should ideally be osteogenic, osteoinductive, osteoconductive, and facilitate vascular in-growth. Autologous bone graft remains the gold standard, providing all of these qualities. Limitations to this technique include low graft volume and donor site morbidity, with alternative techniques including the use of allograft or xenograft. Artificial scaffolds can provide an osteoconductive construct, however fail to provide an osteoinductive stimulus, and frequently have poor mechanical properties. Recombinant bone morphogenetic proteins can provide an osteoinductive stimulus; however, their licencing is limited and larger studies are required to clarify their role. For recalcitricant non-unions or high-risk cases, the use of composite graft combining the above techniques provides the highest chances of successfully achieving bony union.
Keywords: bone graft; bone substitute; fracture healing; non-union.
Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Figures


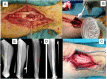
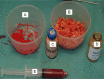
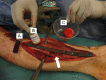


References
-
- Fesseha H & Fesseha Y. Bone grafting, its principle and application: a review. Osteology and Rheumatology 2020143–50. (10.17140/ORHOJ-1-113) - DOI
-
- Schlickewei W & Schlickewei C. The use of bone substitutes in the treatment of bone defects – the clinical view and history. Macromolecular Symposia 200725310–23. (10.1002/masy.200750702) - DOI
Publication types
LinkOut - more resources
Full Text Sources

