Tryptophan metabolism determines outcome in tuberculous meningitis: a targeted metabolomic analysis
- PMID: 37158692
- PMCID: PMC10181821
- DOI: 10.7554/eLife.85307
Tryptophan metabolism determines outcome in tuberculous meningitis: a targeted metabolomic analysis
Abstract
Background: Cellular metabolism is critical for the host immune function against pathogens, and metabolomic analysis may help understand the characteristic immunopathology of tuberculosis. We performed targeted metabolomic analyses in a large cohort of patients with tuberculous meningitis (TBM), the most severe manifestation of tuberculosis, focusing on tryptophan metabolism.
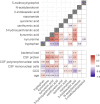
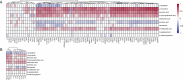
Methods: We studied 1069 Indonesian and Vietnamese adults with TBM (26.6% HIV-positive), 54 non-infectious controls, 50 with bacterial meningitis, and 60 with cryptococcal meningitis. Tryptophan and downstream metabolites were measured in cerebrospinal fluid (CSF) and plasma using targeted liquid chromatography-mass spectrometry. Individual metabolite levels were associated with survival, clinical parameters, CSF bacterial load and 92 CSF inflammatory proteins.
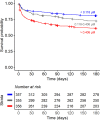
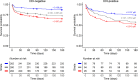
Results: CSF tryptophan was associated with 60-day mortality from TBM (hazard ratio [HR] = 1.16, 95% confidence interval [CI] = 1.10-1.24, for each doubling in CSF tryptophan) both in HIV-negative and -positive patients. CSF tryptophan concentrations did not correlate with CSF bacterial load nor CSF inflammation but were negatively correlated with CSF interferon-gamma concentrations. Unlike tryptophan, CSF concentrations of an intercorrelating cluster of downstream kynurenine metabolites did not predict mortality. These CSF kynurenine metabolites did however correlate with CSF inflammation and markers of blood-CSF leakage, and plasma kynurenine predicted death (HR 1.54, 95% CI = 1.22-1.93). These findings were mostly specific for TBM, although high CSF tryptophan was also associated with mortality from cryptococcal meningitis.
Conclusions: TBM patients with a high baseline CSF tryptophan or high systemic (plasma) kynurenine are at increased risk of death. These findings may reveal new targets for host-directed therapy.
Funding: This study was supported by National Institutes of Health (R01AI145781) and the Wellcome Trust (110179/Z/15/Z and 206724/Z/17/Z).
Keywords: central nervous system; cerebrospinal fluid; human; immunology; inflammation; metabolomics; plasma; survival; tuberculous meningitis.
© 2023, Ardiansyah et al.
Conflict of interest statement
EA, JA, LN, SD, DV, HH, KB, BA, RE, TT, JD, DH, TC, NB, AG, RR, VK, RH, DI, KM, VK, CC, Rv, GT, Av, NT No competing interests declared, MN has received consulting fees from Scientific Board TTxD and is a scientific founder of TTxD, Lemba and BioTRIP. The author has no other competing interests to declare
Figures









Update of
-
Tryptophan metabolism determines outcome in tuberculous meningitis: a targeted metabolomic analysis.medRxiv [Preprint]. 2023 Jan 9:2023.01.08.23284316. doi: 10.1101/2023.01.08.23284316. medRxiv. 2023. Update in: Elife. 2023 May 09;12:e85307. doi: 10.7554/eLife.85307. PMID: 36711829 Free PMC article. Updated. Preprint.
References
-
- Assarsson E, Lundberg M, Holmquist G, Björkesten J, Thorsen SB, Ekman D, Eriksson A, Rennel Dickens E, Ohlsson S, Edfeldt G, Andersson A-C, Lindstedt P, Stenvang J, Gullberg M, Fredriksson S. Homogenous 96-plex pea immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLOS ONE. 2014;9:e95192. doi: 10.1371/journal.pone.0095192. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

