First-in-human phase I/Ib study of QL1706 (PSB205), a bifunctional PD1/CTLA4 dual blocker, in patients with advanced solid tumors
- PMID: 37158938
- PMCID: PMC10169367
- DOI: 10.1186/s13045-023-01445-1
First-in-human phase I/Ib study of QL1706 (PSB205), a bifunctional PD1/CTLA4 dual blocker, in patients with advanced solid tumors
Abstract
Background: QL1706 (PSB205) is a single bifunctional MabPair (a novel technical platform) product consisting of two engineered monoclonal antibodies (anti-PD-1 IgG4 and anti-CTLA-4 IgG1), with a shorter elimination half-life (t1/2) for CTLA-4. We report results from a phase I/Ib study of QL1706 in patients with advanced solid tumors who failed standard therapies.
Methods: In the phase I study, QL1706 was administered intravenously once every 3 weeks at one of five doses ranging from 0.3 to 10 mg/kg, and the maximum tolerated dose, recommended phase 2 dose (RP2D), safety, pharmacokinetics (PK), and pharmacodynamics (PD) of QL1706 were investigated. In the phase Ib study, QL1706 was administered at the RP2D intravenously every 3 weeks, and the preliminary efficacies in non-small cell lung cancer (NSCLC), nasopharyngeal carcinoma (NPC), cervical cancer (CC), and other solid tumors were evaluated.
Results: Between March 2020 and July 2021, 518 patients with advanced solid tumors were enrolled (phase I, n = 99; phase Ib, n = 419). For all patients, the three most common treatment-related adverse events (TRAEs) were rash (19.7%), hypothyroidism (13.5%), and pruritus (13.3%). The TRAEs and immune-related adverse events (irAEs) of grade ≥ 3 occurred in 16.0% and 8.1% of patients, respectively. In phase I, 2 of 6 patients in the 10mg/kg group experienced dose-limiting toxicities (DLTs) (grade 3 thrombocytopenia and grade 4 immune-mediated nephritis), so the maximum tolerated dose (MTD) was reached at 10 mg/kg. The RP2D was determined to be 5 mg/kg based on comprehensive analysis of tolerability, PK/PD, and efficacy. For all patients who received QL1706 at the RP2D, the objective response rate (ORR) and median duration of response were 16.9% (79/468) and 11.7 months (8.3-not reached [NR]), respectively; and the ORRs were 14.0% (17/121) in NSCLC, 24.5% (27/110) in NPC, 27.3% (15/55) in CC, 7.4% (2/27) in colorectal cancer, 23.1% (6/26) in small cell lung cancer. For immunotherapy-naive patients, QL1706 exhibited promising antitumor activities, especially in NSCLC, NPC, and CC, with ORRs of 24.2%, 38.7%, and 28.3%, respectively.
Conclusions: QL1706 was well tolerated and demonstrated promising antitumor activity in solid tumors, especially in NSCLC, NPC, and CC patients. It is currently being evaluated in randomized phase II (NCT05576272, NCT05179317) and phase III (NCT05446883, NCT05487391) trials. Trial Registration ClinicalTrials.gov Identifier: NCT04296994 and NCT05171790.
Keywords: Bifunctional PD-1; CTLA4 antibody; Cervical cancer; MabPair; Nasopharyngeal carcinoma; Phase I trial.
© 2023. The Author(s).
Conflict of interest statement
Li Zhang reports receiving research support from Jiangsu Hengrui Pharmaceuticals, Eli Lilly, Novartis, Roche, and Bristol-Myers Squibb. Zhi Liu, Yufeng Peng, Bill Fanslow, Xian Huang, and Wei Yan are employees of Sound Biologics. Xiaoyan Kang, Shilin Xue, Guihong Yang, and Yingying Yang are employees of Qilu Pharma Ltd. All other authors declare no potential competing interests.
Figures
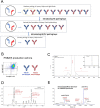

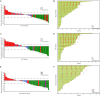

References
-
- Pires da Silva I, Ahmed T, Reijers ILM, Weppler AM, Betof Warner A, Patrinely JR, et al. Ipilimumab alone or ipilimumab plus anti-PD-1 therapy in patients with metastatic melanoma resistant to anti-PD-(L)1 monotherapy: a multicentre, retrospective, cohort study. Lancet Oncol. 2021;22:836–847. doi: 10.1016/S1470-2045(21)00097-8. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

