Condensation of the β-cell secretory granule luminal cargoes pro/insulin and ICA512 RESP18 homology domain
- PMID: 37159024
- PMCID: PMC10201709
- DOI: 10.1002/pro.4649
Condensation of the β-cell secretory granule luminal cargoes pro/insulin and ICA512 RESP18 homology domain
Abstract
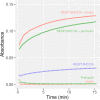
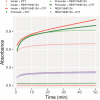
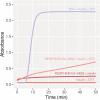
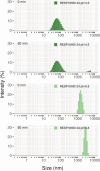
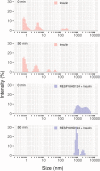
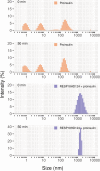
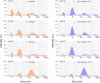
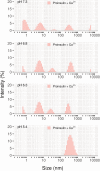
ICA512/PTPRN is a receptor tyrosine-like phosphatase implicated in the biogenesis and turnover of the insulin secretory granules (SGs) in pancreatic islet beta cells. Previously we found biophysical evidence that its luminal RESP18 homology domain (RESP18HD) forms a biomolecular condensate and interacts with insulin in vitro at close-to-neutral pH, that is, in conditions resembling those present in the early secretory pathway. Here we provide further evidence for the relevance of these findings by showing that at pH 6.8 RESP18HD interacts also with proinsulin-the physiological insulin precursor found in the early secretory pathway and the major luminal cargo of β-cell nascent SGs. Our light scattering analyses indicate that RESP18HD and proinsulin, but also insulin, populate nanocondensates ranging in size from 15 to 300 nm and 10e2 to 10e6 molecules. Co-condensation of RESP18HD with proinsulin/insulin transforms the initial nanocondensates into microcondensates (size >1 μm). The intrinsic tendency of proinsulin to self-condensate implies that, in the ER, a chaperoning mechanism must arrest its spontaneous intermolecular condensation to allow for proper intramolecular folding. These data further suggest that proinsulin is an early driver of insulin SG biogenesis, in a process in which its co-condensation with RESP18HD participates in their phase separation from other secretory proteins in transit through the same compartments but destined to other routes. Through the cytosolic tail of ICA512, proinsulin co-condensation with RESP18HD may further orchestrate the recruitment of cytosolic factors involved in membrane budding and fission of transport vesicles and nascent SGs.
Keywords: insulin; mesoscopic clusters; nanocondensates; proinsulin; protein secretion; protein sorting; protein tyrosine phosphatase; protein-protein interactions; secretory granule biogenesis; β cell.
© 2023 The Authors. Protein Science published by Wiley Periodicals LLC on behalf of The Protein Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Arnaoutova I, Smith AM, Coates LC, Sharpe JC, Dhanvantari S, Snell CR, et al. The prohormone processing enzyme PC3 is a lipid raft‐associated transmembrane protein. Biochemistry. 2003;42:10445–55. - PubMed
-
- Arvan P, Halban PA. Sorting ourselves out: seeking consensus on trafficking in the beta‐cell. Traffic. 2004;5:53–61. - PubMed
-
- Assadi M, Sharpe JC, Snell C, Loh YP. The C‐terminus of prohormone convertase 2 is sufficient and necessary for raft association and sorting to the regulated secretory pathway. Biochemistry. 2004;43:7798–807. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

