Effect of Prophylactic Antibiotics on Mortality in Severe Alcohol-Related Hepatitis: A Randomized Clinical Trial
- PMID: 37159035
- PMCID: PMC10170332
- DOI: 10.1001/jama.2023.4902
Effect of Prophylactic Antibiotics on Mortality in Severe Alcohol-Related Hepatitis: A Randomized Clinical Trial
Abstract
Importance: The benefits of prophylactic antibiotics for hospitalized patients with severe alcohol-related hepatitis are unclear.
Objective: To determine the efficacy of amoxicillin-clavulanate, compared with placebo, on mortality in patients hospitalized with severe alcohol-related hepatitis and treated with prednisolone.
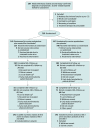
Design, setting, and participants: Multicenter, randomized, double-blind clinical trial among patients with biopsy-proven severe alcohol-related hepatitis (Maddrey function score ≥32 and Model for End-stage Liver Disease [MELD] score ≥21) from June 13, 2015, to May 24, 2019, in 25 centers in France and Belgium. All patients were followed up for 180 days. Final follow-up occurred on November 19, 2019.
Intervention: Patients were randomly assigned (1:1 allocation) to receive prednisolone combined with amoxicillin-clavulanate (n = 145) or prednisolone combined with placebo (n = 147).
Main outcome and measures: The primary outcome was all-cause mortality at 60 days. Secondary outcomes were all-cause mortality at 90 and 180 days; incidence of infection, incidence of hepatorenal syndrome, and proportion of participants with a MELD score less than 17 at 60 days; and proportion of patients with a Lille score less than 0.45 at 7 days.
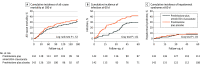
Results: Among 292 randomized patients (mean age, 52.8 [SD, 9.2] years; 80 [27.4%] women) 284 (97%) were analyzed. There was no significant difference in 60-day mortality between participants randomized to amoxicillin-clavulanate and those randomized to placebo (17.3% in the amoxicillin-clavulanate group and 21.3% in the placebo group [P = .33]; between-group difference, -4.7% [95% CI, -14.0% to 4.7%]; hazard ratio, 0.77 [95% CI, 0.45-1.31]). Infection rates at 60 days were significantly lower in the amoxicillin-clavulanate group (29.7% vs 41.5%; mean difference, -11.8% [95% CI, -23.0% to -0.7%]; subhazard ratio, 0.62; [95% CI, 0.41-0.91]; P = .02). There were no significant differences in any of the remaining 3 secondary outcomes. The most common serious adverse events were related to liver failure (25 in the amoxicillin-clavulanate group and 20 in the placebo group), infections (23 in the amoxicillin-clavulanate group and 46 in the placebo group), and gastrointestinal disorders (15 in the amoxicillin-clavulanate group and 21 in the placebo group).
Conclusion and relevance: In patients hospitalized with severe alcohol-related hepatitis, amoxicillin-clavulanate combined with prednisolone did not improve 2-month survival compared with prednisolone alone. These results do not support prophylactic antibiotics to improve survival in patients hospitalized with severe alcohol-related hepatitis.
Trial registration: ClinicalTrials.gov Identifier: NCT02281929.
Conflict of interest statement
Figures
Comment in
-
The Role of Prophylactic Antibiotics for Patients With Severe Alcohol-Related Hepatitis.JAMA. 2023 May 9;329(18):1552-1553. doi: 10.1001/jama.2023.1729. JAMA. 2023. PMID: 37159045 No abstract available.
References
-
- Louvet A, Thursz MR, Kim DJ, et al. . Corticosteroids reduce risk of death within 28 days for patients with severe alcoholic hepatitis, compared with pentoxifylline or placebo—a meta-analysis of individual data from controlled trials. Gastroenterology. 2018;155(2):458-468. doi:10.1053/j.gastro.2018.05.011 - DOI - PubMed

