Treatment for osteoporosis in people with beta-thalassaemia
- PMID: 37159055
- PMCID: PMC10167785
- DOI: 10.1002/14651858.CD010429.pub3
Treatment for osteoporosis in people with beta-thalassaemia
Abstract
Background: Osteoporosis is characterized by low bone mass and micro-architectural deterioration of bone tissue leading to increased bone fragility. In people with beta-thalassaemia, osteoporosis represents an important cause of morbidity and is due to a number of factors. First, ineffective erythropoiesis causes bone marrow expansion, leading to reduced trabecular bone tissue with cortical thinning. Second, excessive iron loading causes endocrine dysfunction, leading to increased bone turnover. Lastly, disease complications can result in physical inactivity, with a subsequent reduction in optimal bone mineralization. Treatments for osteoporosis in people with beta-thalassaemia include bisphosphonates (e.g. clodronate, pamidronate, alendronate; with or without hormone replacement therapy (HRT)), calcitonin, calcium, zinc supplementation, hydroxyurea, and HRT alone (for preventing hypogonadism). Denosumab, a fully human monoclonal antibody, inhibits bone resorption and increases bone mineral density (BMD). Finally, strontium ranelate simultaneously promotes bone formation and inhibits bone resorption, thus contributing to a net gain in BMD, increased bone strength, and reduced fracture risk. This is an update of a previously published Cochrane Review.
Objectives: To review the evidence on the efficacy and safety of treatment for osteoporosis in people with beta-thalassaemia.
Search methods: We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group's Haemoglobinopathies Trials Register, which includes references identified from comprehensive electronic database searches and handsearches of relevant journals and abstract books of conference proceedings. We also searched online trial registries. Date of most recent search: 4 August 2022.
Selection criteria: Randomized controlled trials (RCTs) in people with beta-thalassaemia with: a BMD Z score below -2 standard deviations (SDs) for children aged under 15 years, adult males (aged 15 to 50 years) and premenopausal females aged over 15 years; or a BMD T score below -2.5 SDs for postmenopausal females and males aged over 50 years.
Data collection and analysis: Two review authors assessed the eligibility and risk of bias of the included RCTs, and extracted and analysed data. We assessed the certainty of the evidence using GRADE.
Main results: We included six RCTs (298 participants). Active interventions included bisphosphonates (3 trials, 169 participants), zinc supplementation (1 trial, 42 participants), denosumab (1 trial, 63 participants), and strontium ranelate (1 trial, 24 participants). The certainty of the evidence ranged from moderate to very low and was downgraded mainly due to concerns surrounding imprecision (low participant numbers), but also risk of bias issues related to randomization, allocation concealment, and blinding. Bisphosphonates versus placebo or no treatment Two RCTs compared bisphosphonates to placebo or no treatment. After two years, one trial (25 participants) found that alendronate and clodronate may increase BMD Z score compared to placebo at the femoral neck (mean difference (MD) 0.40, 95% confidence interval (CI) 0.22 to 0.58) and the lumbar spine (MD 0.14, 95% CI 0.05 to 0.23). One trial (118 participants) reported that neridronate compared to no treatment may increase BMD at the lumbar spine and total hip at six and 12 months; for the femoral neck, the study found increased BMD in the neridronate group at 12 months only. All results were of very low-certainty. There were no major adverse effects of treatment. Participants in the neridronate group reported less back pain; we considered this representative of improved quality of life (QoL), though the certainty of the evidence was very low. One participant in the neridronate trial (116 participants) sustained multiple fractures as a result of a traffic accident. No trials reported BMD at the wrist or mobility. Different doses of bisphosphonate compared One 12-month trial (26 participants) assessed different doses of pamidronate (60 mg versus 30 mg) and found a difference in BMD Z score favouring the 60 mg dose at the lumbar spine (MD 0.43, 95% CI 0.10 to 0.76) and forearm (MD 0.87, 95% CI 0.23 to 1.51), but no difference at the femoral neck (very low-certainty evidence). This trial did not report fracture incidence, mobility, QoL, or adverse effects of treatment. Zinc versus placebo One trial (42 participants) showed zinc supplementation probably increased BMD Z score compared to placebo at the lumbar spine after 12 months (MD 0.15, 95% CI 0.10 to 0.20; 37 participants) and 18 months (MD 0.34, 95% CI 0.28 to 0.40; 32 participants); the same was true for BMD at the hip after 12 months (MD 0.15, 95% CI 0.11 to 0.19; 37 participants) and 18 months (MD 0.26, 95% CI 0.21 to 0.31; 32 participants). The evidence for these results was of moderate certainty. The trial did not report BMD at the wrist, fracture incidence, mobility, QoL, or adverse effects of treatment. Denosumab versus placebo Based on one trial (63 participants), we are unsure about the effect of denosumab on BMD Z score at the lumbar spine, femoral neck, and wrist joint after 12 months compared to placebo (low-certainty evidence). This trial did not report fracture incidence, mobility, QoL, or adverse effects of treatment, but the investigators reported a reduction in bone pain measured on a visual analogue scale in the denosumab group after 12 months of treatment compared to placebo (MD -2.40 cm, 95% CI -3.80 to -1.00). Strontium ranelate One trial (24 participants) only narratively reported an increase in BMD Z score at the lumbar spine in the intervention group and no corresponding change in the control group (very low-certainty evidence). This trial also found a reduction in back pain measured on a visual analogue scale after 24 months in the strontium ranelate group compared to the placebo group (MD -0.70 cm (95% CI -1.30 to -0.10); we considered this measure representative of improved quality of life.
Authors' conclusions: Bisphosphonates may increase BMD at the femoral neck, lumbar spine, and forearm compared to placebo after two years' therapy. Zinc supplementation probably increases BMD at the lumbar spine and hip after 12 months. Denosumab may make little or no difference to BMD, and we are uncertain about the effect of strontium on BMD. We recommend further long-term RCTs on different bisphosphonates and zinc supplementation therapies in people with beta-thalassaemia-associated osteoporosis.
Copyright © 2023 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
AB: none known KMMS: none known NKS: none known IO: served on the speakers bureau for Novartis pharmaceuticals for Exjade and Jadenu, providing disease education on sickle cell disease and education to providers and patients on managing iron overload.
Figures
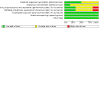















Update of
-
Treatment for osteoporosis in people with ß-thalassaemia.Cochrane Database Syst Rev. 2016 Mar 10;3:CD010429. doi: 10.1002/14651858.CD010429.pub2. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2023 May 9;5:CD010429. doi: 10.1002/14651858.CD010429.pub3. PMID: 26964506 Updated.
References
References to studies included in this review
Forni 2012 {published data only}
-
- Forni GL, Giusti A, Pinto V, Perrotta S, Borgna Pignatti C, D'Ascola DG, et al. Neridronate improves bone mineral density and reduces pain in beta-thalassemia patients with osteoporosis: Results from a randomized, open-label study. Bone 2012;50:S153. [CENTRAL: CN-01746160] [CFGD REGISTER: TH108c] [EMBASE: 71731342] - PubMed
-
- Forni GL, Perrotta S, Borgna Pignatti C, D'Ascola DG, Nobili B, Pitrolo L, et al. Neridronate (NE) for the treatment of osteoporosis in patients with beta-thalassemia: results from an Italian multicenter randomized, open label, phase II trial. Blood 2010;116(21):4282. [ABSTRACT NO.: 4282] [CENTRAL: 848923] [CFGD REGISTER: TH108a]
-
- Forni GL, Perrotta S, Giusti A, Quarta G, Pitrolo L, Cappellini MD, et al. Neridronate improves bone mineral density and reduces back pain in beta-thalassaemia patients with osteoporosis: results from a phase 2, randomized parallel-arm, open-label study. British Journal of Haematology 2012;158(2):274-82. [CFGD REGISTER: TH108b] - PubMed
-
- NCT01140321. Efficacy and safety of neridronate (nerixia®) to treat osteoporosis in patients with TM and TI. www.clinicaltrials.gov/show/NCT01140321 (first posted 9 June 2010). [CFGD REGISTER: TH108d]
Fung 2013 {published data only}
-
- Fung EB, Kwiatkowski JL, Huang JN, Gildengorin G, King JC, Vichinsky EP. Zinc supplementation improves bone density in patients with thalassemia: a double-blind, randomized, placebo-controlled trial. American Journal of Clinical Nutrition 2013;98(4):960-71. [CENTRAL: 876084] [CFGD REGISTER: TH112] [PMID: ] - PMC - PubMed
-
- NCT00459732. Zinc & bone health in thalassemia: the think zinc study. clinicaltrials.gov/show/NCT00459732 (first posted 12 April 2007). [CFGD REGISTER: TH112b]
Morabito 2002 {published data only}
-
- Morabito N, Lasco A, Gaudio A, Crisafulli A, Di Pietro C, Meo A, Frisina N. Bisphosphonates in the treatment of thalassemia-induced osteoporosis. Osteoporosis International 2002;13(8):644-9. [CFGD REGISTER: TH41] - PubMed
Morabito 2016 {published data only}
-
- Catalano A, Morabito N, Gaudio A, Morini E, Basile G, Tsiantouli E, et al. Bone mass and bone turnover in women with thalassemia major related osteoporosis: Effects of strontium ranelate. Osteoporosis International 2015;26(1 Suppl 1):S248. [ABSTRACT NO.: P487] [CENTRAL: 1080008] [CFGD REGISTER: TH141a] [EMBASE: 71877007]
-
- Morabito N, Catalano A, Gaudio A, Morini E, Bruno LM, Basile G, et al. Effects of strontium ranelate on bone mass and bone turnover in women with thalassemia major-related osteoporosis. Journal of Bone and Mineral Metabolism 2016;34(5):540-6. [CFGD REGISTER: TH141b] - PubMed
Voskaridou 2003 {published data only}
-
- Voskaridou E, Terpos E, Spina G, Palermos J, Rahemtulla A, Loutradi A, et al. Effect of pamidronate on bone remodeling in adult patients with beta-thalassemia major and osteoporosis. Bone 2003;5(Suppl 1):S205. [CFGD REGISTER: TH44b]
-
- Voskaridou E, Terpos E, Spina G, Palermos J, Rahemtulla A, Loutradi A, et al. Pamidronate is an effective treatment for osteoporosis in patients with thalassemia major. In: 8th Congress of the European Hematology Association; 2003 June 12-15; Lyon, France. 2003. [ABSTRACT NO.: 1] [CFGD REGISTER: TH44a] - PubMed
Voskaridou 2018 {published data only}
-
- EUCTR2014-000931-18-GR. This study investigates the effect of denosumab treatment compared to treatment with placebo, in patients with thalasemia major and osteoporosis. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2014-000931-18-GR (first registered 3 April 2014). [CFGD REGISTER: TH217]
-
- NCT02559648. Denosumab vs placebo in patients with thalassemia major and osteoporosis. www.clinicaltrials.gov/show/NCT02559648 (first posted 24 September 2015). [CFGD REGISTER: TH167e]
-
- Voskaridou E, Ntanasis-Stathopoulos I, Christoulas D, Sonnleitner L, Papaefstathiou A, Dimopoulou M, et al. Denosumab effects on serum levels of the bone morphogenetic proteins antagonist noggin in patients with transfusion-dependent thalassemia and osteoporosis. Hematology 2019;24(1):318-24. [CENTRAL: CN-01707665] [CFGD REGISTER: TH167d] [PMID: ] - PubMed
-
- Voskaridou E, Ntanasis-Stathopoulos I, Papaefstathiou A, Christoulas D, Dimopoulou M, Repa K, et al. Denosumab in transfusion-dependent thalassemia osteoporosis: a randomized, placebo-controlled, double-blind phase 2b clinical trial. Blood Advances 2018;2(21):2837-47. [CENTRAL: CN-01666219] [CFGD REGISTER: TH167c] [EMBASE: 624947802] [PMID: ] - PMC - PubMed
-
- Voskaridou E, Ntanasis-Stathopoulos J, Christoulas D, Sonnleitner L, Papaefstathiou A, Dimopoulou M, et al. Denosumab effects on serum levels of the bone morphogenic proteins antagonist noggin in patients with beta-thalassemia major and osteoporosis. Hemasphere 2018;2(S1):333. [CENTRAL: CN-01790516] [CFGD REGISTER: TH167b] [EMBASE: 625922698]
References to studies excluded from this review
Balachandar 2012 {published data only}
-
- Balachandar S, Randolph R, Kleinert DA, Sheth S, Giardina P, Vigiatzi MG. The effect of vitamin D supplementation on calcium excretion in thalassemia [abstract]. Blood 2012;120:Abstract no: 1029.
Canartan 1995 {published data only}
-
- Canatan D, Akar N, Arcasoy A. Effects of calcitonin therapy on osteoporosis in patients. Acta Haematologica 1995;93:20-4. - PubMed
Chae 2009 {published data only}
-
- Chae YS, Kim JG, Moon JH, Kim SN, Lee SJ, Kim YJ, et al. Pilot study on the use of zoledronic acid to prevent bone loss in allo-SCT recipients. Bone Marrow Transplantation 2009;44:35-41. - PubMed
Chatterjee 2012 {published data only}
-
- Chatterjee R, Shah FT, Davis BA, Byers M, Sooranna D, Bajoria R, et al. Prospective study of histomorphometry, biochemical bone markers and bone densitometric response to pamidronate in beta-thalassaemia presenting with osteopenia-osteoporosis syndrome. British Journal of Haematology 2012;159(4):462-71. [CFGD REGISTER: TH166] - PubMed
Darvish‐Khezri 2018 {published data only}
Gilfillan 2006 {published data only}
-
- Gilfillan CP, Strauss BJ, Rodda CP, Bowden DK, Kean AM, Obaid M, et al. A randomized, double-blind, placebo-controlled trial of intravenous zoledronic acid in the treatment of thalassemia-associated osteopenia. Calcified Tissue International 2006;79(3):138-44. - PubMed
Gurkan 2005 {published data only}
-
- Gurkan EG, Evran M, Tuli A, Baslamisli F, Kilinc Y. Effect of zoledronic acid on the osteoporosis of thalassemia. In: Proceedings of the 10th Congress of the European Hematology Association; 2005 June 2-5; Stockholm International Fairs, Sweden. 2005. [ABSTRACT NO.: 0164]
Krishnan 1994 {published data only}
-
- Krishnan S, Sturtridge WC, Olivieri NF, Collins AF, Qureshi RY, Krishnan M, et al. A study on children's condition thalassemia using neutron activation analysis and other techniques. Biological Trace Elements Research 1994;43(4):309-14. - PubMed
Naithani 2018 {published data only}
Noroozi 2022 {published data only}
-
- Noroozi M, Ghazizadeh F, Gitifar S. Effect of low dose pamidronate in the treatment of thalassemia-induced osteoporosis. Immunopathologia Persa 2022;x:x. [DOI: 10.34172/ipp.2022.xx] - DOI
Olgun 2019 {published data only}
-
- Olgun ME, Gürkan E, Tuli A. Osteoporosis in thalassemia patients and use of zoledronic acid in the treatment of osteoporosis. Cukurova Medical Journal 2019;44(3):882-90.
Otrock 2006 {published data only}
-
- Otrock ZK, Azar ST, Shamseddeen WA, Habr D, Inati A, Koussa S, et al. Intravenous zoledronic acid treatment in thalassemia-induced osteoporosis: results of a phase II clinical trial. Annals of Hematology 2006;85(9):605-9. - PubMed
-
- Taher A, Otrock Z, Azar S, Shamseddeen W, Habr D, Inati A, et al. Intravenous zoledronic acid treatment in thalassaemia-induced osteoporosis: results of a phase two clinical trial [abstract]. Haematologica 2006;91(Suppl 1):10. [CENTRAL: 593094] - PubMed
Pennisi 2003 {published data only}
-
- Pennisi P, Pizzarelli G, Spina M, Riccobene S, Fiore CE. Quantitative ultrasound of bone and clodronate effects in thalassemia-induced osteoporosis. Journal of Bone and Mineral Metabolism 2003;21(6):402-8. - PubMed
Skordis 2008 {published data only}
-
- Skordis N, Ioannou YS, Kyriakou A, Savva SC, Efstathiou E, Savvides I, et al. Effect of bisphosphonate treatment on bone mineral density in patients with thalassaemia major. Pediatric Endocrinology Reviews: PER 2008;6 Suppl 1:144-8. [CENTRAL: 699706] [PMID: ] - PubMed
Voskaridou 2008 {published data only}
-
- Voskaridou E, Christoulas D, Antoniadou L, Tsaftaridis P, Plata E, Terpos E. Zoledronic acid increases bone mineral density in patients with thalassemia intermedia-induced osteoporosis despite the continuous bone marrow expansion. Haematologica 2007;92 Suppl 1:342. [CENTRAL: 625349] [CFGD REGISTER: TH96c]
-
- Voskaridou E, Christoulas D, Antoniadou L, Tsaftaridis T, Plata E, Terpos E. Zoledronic acid increases bone mineral density in patients with thalassemia intermedia-induced osteoporosis regardless of the incessant bone marrow expansion. Blood 2007;110(11):3813. [ABSTRACT NO.: 3813] [CENTRAL: 625484] [CFGD REGISTER: TH96a]
-
- Voskaridou E, Christoulas D, Antoniadou L, Terpos E. Continuous increase in erythropoietic activity despite the improvement in bone mineral density by zoledronic acid in patients with thalassemia intermedia-induced osteoporosis. Acta Haematologica 2008;119(1):40-4. [CFGD REGISTER: TH96b] - PubMed
Voskaridou 2009 {published data only}TH74
-
- NCT00346242. Evaluation of efficacy of zoledronic acid in patients with haemoglobin syndromes (thalassemia and sicle cell anaemia) and risk of skeletal events. clinicaltrials.gov/show/NCT00346242 (first posted 29 June 2006). [CFGD REGISTER: TH74L]
-
- Voskaridou E, Anagnostopoulos A, Konstantopoulos K, Stoupa E, Spyropoulou E, Kiamouris C, et al. Zoledronic acid for the treatment of osteoporosis in patients with beta-thalassemia: results from a single-center, randomized, placebo-controlled trial. Haematologica 2006;91(9):1193-202. [CFGD REGISTER: TH74b] - PubMed
-
- Voskaridou E, Christoulas D, Konstantinidou M, Tsiftsakis E, Alexakos P, Terpos E. Continuous improvement of bone mineral density two years post zoledronic acid discontinuation in patients with thalassemia-induced osteoporosis: long-term follow-up of a randomized, placebo-controlled trial. Haematologica 2008;93(10):1588-90. [CFGD REGISTER: TH74i] - PubMed
-
- Voskaridou E, Christoulas D, Konstantinidou M, Tsiftsakis E, Spyropoulou E, Alexakos P, et al. Continuous improvement of bone mineral density two years post zoledronic acid discontinuation in patients with thalassemia-induced osteoporosis: long-term follow- up of a randomized, placebo-controlled trial. Blood 2007;110(11):2768. [ABSTRACT NO.: 2768] [CFGD REGISTER: TH74c] - PubMed
-
- Voskaridou E, Christoulas D, Pantelaros T, Varvagiannis K, Xirakia C, Papatheodorou A, et al. Serum Dickkopf-1 is increased and correlates with bone mineral density in patients with thalassemia-induced osteoporosis. Reduction post-zoledronic acid administration. Blood 2008;112(11):3889. [ABSTRACT NO.: 3889] [CFGD REGISTER: TH74e] - PMC - PubMed
Yassin 2020 {published data only}
-
- NCT03040765. Denosumab versus zoledronic acid in thalassemia-induced osteoporosis. www.clinicaltrials.gov/show/NCT03040765 (first posted 2 February 2017). [CFGD REGISTER: TH216a]
References to studies awaiting assessment
Eid 2021 {published data only}
-
- Eid MA, Aly SM. Effect of whole body vibration training on bone mineral density and functional capacity in children with thalassemia. Physiotherapy Theory and Practice 2021;37(2):279-86. [CFGD REGISTER: TH231b] - PubMed
-
- PACTR201804003248173. Effect of whole body vibration training on bone mineral density and functional capacity in children with thalassemia. trialsearch.who.int/Trial2.aspx?TrialID=PACTR201804003248173 (first registered 24 March 2018). [CFGD REGISTER: TH231a]
TCTR20201223008 {published data only}
-
- TCTR20201223008. Effects of melatonin on bone metabolism in thalassemia patients with low bone mineral density (randomized controlled study). trialsearch.who.int/Trial2.aspx?TrialID=TCTR20201223008 (first registered 23 December 2020). [CFGD REGISTER: TH233]
References to ongoing studies
CTRI/2019/04/018764 {published data only}
-
- CTRI/2019/04/018764. Trial to study the role of zoledronate in the prevention of early bone loss in patients undergoing bone marrow transplant. in prevention of early bone loss in patients undergoing bone marrow transplant. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2019/04/018764 (first registered 24 April 2019). [CFGD REGISTER: TH232]
IRCT2017070420258N51 {published data only}
-
- IRCT2017070420258N51. Evaluation of intravenous pamidronate & oral alendronate on bone marrow density in patients with major and intermedia thalassemia. trialsearch.who.int/Trial2.aspx?TrialID=IRCT2017070420258N51 (first registered 16 July 2017). [CFGD REGISTER: TH230]
NCT01016093 {published data only}
-
- NCT01016093. Zoledronic acid for the prevention of bone loss post-bone marrow transplantation for thalassemia major patients. clinicaltrials.gov/show/NCT01016093 (first posted 18 November 2009). [CFGD REGISTER: TH229]
Piriyakhuntorn 2019 {published data only}
-
- Piriyakhuntorn P, Tantiworawit A, Phimphilai M, Srichairatanakool S, Teeyasoontranon W, Rattanathammethee T, et al. The efficacy of alendronate for the treatment of thalassemia-associated osteoporosis: a randomized controlled trial. Hemasphere 2019;3 Suppl 1:347-8. [CFGD REGISTER: TH218c] - PMC - PubMed
-
- Piriyakhuntorn P, Tantiworawit A, Phimphilai M, Srichairatanakool S, Teeyasoontranon W, Rattanathammethee T, et al. The efficacy of alendronate for treatment of thalassemia-associated osteoporosis: a randomized controlled trial. In: 35th Annual Meeting The Royal College of Physicians of Thailand. Towards better and safer patient care; 2019 April 25 - 27; PEACH Royal Cliff Beach Resort, Pattaya, Chonburi, Thailand. 2019. [CFGD REGISTER: TH218b]
-
- TCTR20180219004. Efficacy of alendronate in treatment of thalassemia-associated osteoporosis: a randomized controlled trial. trialsearch.who.int/Trial2.aspx?TrialID=TCTR20180219004 (date first registered 19 Feb 2018). [CFGD REGISTER: TH218a]
Additional references
Akesson 2003
Al‐Refaie 1994
Anapliotou 1995
-
- Anapliotou ML, Kastanias IT, Psara P, Evangelou EA, Liparaki M, Dimitriou P. The contribution of hypogonadism to the development of osteoporosis in thalassaemia major: new therapeutic approaches. Clinical Endocrinology 1995;42(3):279-87. - PubMed
Angastiniotis 1998
-
- Angastiniotis M, Modell B. Global epidemiology of hemoglobin disorders. Annals of the New York Academy of Sciences 1998;850:251-69. - PubMed
Arcasoy 1987
-
- Arcasoy A, Cavdar A, Cin S, Erten J, Babacan E, Gözdasoglu S, et al. Effect of zinc supplementation on linear growth in thalassaemia. American Journal of Hematology 1987;24(2):127-36. - PubMed
Athanasios 2007
-
- Christoforidis A, Kazantzidou E, Tsatra I, Tsantali H, Koliakos G, Hatzipantelis E, et al. Normal lumbar bone mineral density in optimally treated children and young adolescents with β-thalassaemia major. Hormones 2007;6(4):334-40. - PubMed
Basanagoudar 2001
-
- Basanagoudar PL, Gill SS, Dhillon MS, Marwaha RK. Fractures in transfusion dependent beta thalassemia – an Indian study. Singapore Medical Journal 2001;42(5):196-9. - PubMed
Bordbhar 2014
Borgna‐Pignatti 2007
-
- Borgna-Pignatti C. Modern treatment of thalassaemia intermedia. British Journal of Haematology 2007;138(3):291-304. - PubMed
Canatan 1995
-
- Canatan D, Akar N, Arcasoy A. Effects of calcitonin therapy on osteoporosis in patients with thalassemia. Acta Haematologica 1995;93(1):20-4. - PubMed
Christoforidis 2007
-
- Christoforidis A, Kazantzidou E, Tsatra I, Tsantali H, Koliakos G, Hatzipantelis E, et al. Normal lumbar bone mineral density in optimally treated children and young adolescents with beta-thalassaemia major. Hormone 2007;6(4):334-40. - PubMed
Cummings 2009
-
- Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. New England Journal of Medicine 2009;361(8):756-65. - PubMed
De Sanctis 1996
-
- De Sanctis V, Pinamonti A, Di Palma A, Sprocati M, Atti G, Gamberini MR, et al. Growth and development in thalassaemia major patients with bone lesion due to desferrioxamine. European Journal of Pediatrics 1996;155(5):368-72. - PubMed
Deeks 2022
-
- Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Donner 2001
-
- Donner A, Piaggio G, Villar J. Statistical methods for the meta-analysis of cluster randomization trials. Statistical Methods in Medical Research 2001;10(5):325-8. - PubMed
Elbourne 2002
-
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analysis involving cross-over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140-9. - PubMed
Fleisch 1997
-
- Fleisch H. Mechanisms of action of the bisphosphonates. Medicina (Buenos Aires) 1997;57 Suppl 1:65-75. - PubMed
Galanello 2010
Gaudio 2008
-
- Gaudio A, Morabito N, Xourafa A, Macrì I, Meo A, Morgante S, et al. Bisphosphonates in the treatment of thalassemia-associated osteoporosis. Journal of Endocrinological Investigation 2008;31(2):181-4. - PubMed
Higgins 2017
-
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Jensen 1998a
-
- Jensen CE, Tuck SM, Agnew JE, Koneru S, Morris RW, Yardumian A, et al. High incidence of osteoporosis in thalassaemia major. Journal of Pediatric Endocrinology and Metabolism 1998;11(3):975-7. - PubMed
Jensen 1998b
-
- Jensen CE, Tuck SM, Agnew JE, Koneru S, Morris RW, Yardumian A, et al. High prevalence of low bone mass in thalassaemia major. British Journal of Haematology 1998;103(4):911-5. - PubMed
Lindsay 1993
-
- Lindsay R. Hormone replacement therapy for prevention and treatment of osteoporosis. American Journal of Medicine 1993;95(5A):37S-39S. - PubMed
Mahyar 2010
Mokhtar 2011
-
- Mokhtar GM, Tantawy AA, Adly AA, Ismail EA. Clinicopathological and radiological study of Egyptian β-thalassemia intermedia and β-thalassemia major patients: relation to complications and response to therapy. Hemoglobin 2011;35(4):382-405. - PubMed
Papapoulus 2015
Perifanis 2004
-
- Perifanis V, Vyzantiadis T, Vakalopoulou S, Tziomalos K, Garypidou V, Athanassiou-Metaxa M, et al. Treatment of beta-thalassaemia-associated osteoporosis with zoledronic acid. British Journal of Haematology 2004;125(1):91-2. - PubMed
RevMan 2014 [Computer program]
-
- Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ruggierol 1998
-
- Ruggiero L, De Sanctis V. Multicentre study on prevalence of fractures in transfusion-dependent thalassaemic patients. Journal of Pediatric Endocrinology & Metabolism 1998;11(Suppl 3):3773-8. - PubMed
Sanctis 2013
-
- De Sanctis V, Soliman AT, Elsedfy H, Yassin M, Canatan D, Kilinc Y, et al. Osteoporosis in thalassemia major: an update and the I-CET 2013 recommendations for surveillance and treatment. Pediatric Endocrinology Reviews 2013;11(2):167-80. - PubMed
Scacchi 2008
-
- Scacchi M, Danesi L, Cattaneo A, Valassi E, Pecori Giraldi F, Argento C, et al. Bone demineralization in adult thalassaemic patients: contribution of GH and IGF-I at different skeletal sites. Clinical Endocrinology 2008;69(2):202-7. - PubMed
Shamshirsaz 2007
-
- Shamshirsaz AA, Bekheirnia MR, Kamgar M, Pakbaz Z, Tabatabaie SM, Bouzari N, et al. Bone mineral density in Iranian adolescents and young adults with beta-thalassemia major. Pediatric Hematology and Oncology 2007;24(7):469-79. - PubMed
Suda 1997
-
- Suda T, Nakamura I, Jimi E, Takahashi N. Regulation of osteoclast function. Journal of Bone and Mineral Research 1997;12(6):869-79. - PubMed
Sugimoto 2015
-
- Sugimoto T, Matsumoto T, Hosoi T, et al. Three-year denosumab treatment in postmenopausal Japanese women and men with osteoporosis: results from a 1-year open-label extension of the Denosumab Fracture Intervention Randomized Placebo Controlled Trial (DIRECT). Osteoporos International 2015;26(2):765-74. - PubMed
Sutipornpalangkul 2010
-
- Sutipornpalangkul W, Janechetsadatham Y, Siritanaratkul N, Harnroongroj T. Prevalence of fractures among Thais with thalassaemia syndromes. Singapore Medical Journal 2010;51(10):817-21. - PubMed
Swe 2013
Vichinsky 1998
-
- Vichinsky EP. The morbidity of bone disease in thalassaemia. Annals New York Academy of Sciences 1998;850:344-8. - PubMed
Vogiatzi 2006
Voskaridou 2004
-
- Voskaridou E, Terpos E. New insight into the pathophysiology and management of osteoporosis in patient with beta thalassaemia. British Journal of Haematology 2004;127(2):127-39. - PubMed
Weatherall 1995
-
- Weatherall DJ. The Thalassemias. In: Beutler E, Lichtman MA, Coller BS, Kipps TJ, editors(s). Williams Hematology. 5th edition. McGraw-Hill Book Company, 1995:581-615.
WHO 1994
-
- World Health Organization. Assessment of fracture risk and its implication to screening for postmenopausal osteoporosis: Technical report series 843. whqlibdoc.who.int/trs/WHO_TRS_843.pdf (accessed 4 January 2012).
Wonke 1998
-
- Wonke B. Bone disease in β-thalassaemia major. British Journal of Haematology 1998;103(4):897-901. - PubMed
Wonke 2001
-
- Wonke B. Clinical management of beta-thalassemia major. Seminars in Hematology 2001;38(4):350-9. - PubMed
References to other published versions of this review
Bhardwaj 2013
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

