Human T cells efficiently control RSV infection
- PMID: 37159271
- PMCID: PMC10393221
- DOI: 10.1172/jci.insight.168110
Human T cells efficiently control RSV infection
Abstract
Respiratory syncytial virus (RSV) infection causes significant morbidity and mortality in infants, immunocompromised individuals, and older individuals. There is an urgent need for effective antivirals and vaccines for high-risk individuals. We used 2 complementary in vivo models to analyze RSV-associated human lung pathology and human immune correlates of protection. RSV infection resulted in widespread human lung epithelial damage, a proinflammatory innate immune response, and elicited a natural adaptive human immune response that conferred protective immunity. We demonstrated a key role for human T cells in controlling RSV infection. Specifically, primed human CD8+ T cells or CD4+ T cells effectively and independently control RSV replication in human lung tissue in the absence of an RSV-specific antibody response. These preclinical data support the development of RSV vaccines, which also elicit effective T cell responses to improve RSV vaccine efficacy.
Keywords: Adaptive immunity; Immunology; Mouse models; T cells; Virology.
Figures
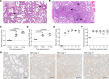

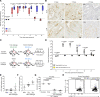


References
-
- Shi T, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 2017;390(10098):946–958. doi: 10.1016/S0140-6736(17)30938-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

