Patient-tailored adoptive immunotherapy with EBV-specific T cells from related and unrelated donors
- PMID: 37159273
- PMCID: PMC10266790
- DOI: 10.1172/JCI163548
Patient-tailored adoptive immunotherapy with EBV-specific T cells from related and unrelated donors
Abstract
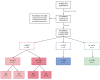
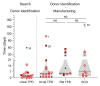
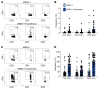
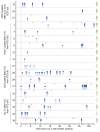
BACKGROUNDAdoptive transfer of EBV-specific T cells can restore specific immunity in immunocompromised patients with EBV-associated complications.METHODSWe provide results of a personalized T cell manufacturing program evaluating donor, patient, T cell product, and outcome data. Patient-tailored clinical-grade EBV-specific cytotoxic T lymphocyte (EBV-CTL) products from stem cell donors (SCDs), related third-party donors (TPDs), or unrelated TPDs from the allogeneic T cell donor registry (alloCELL) at Hannover Medical School were manufactured by immunomagnetic selection using a CliniMACS Plus or Prodigy device and the EBV PepTivators EBNA-1 and Select. Consecutive manufacturing processes were evaluated, and patient outcome and side effects were retrieved by retrospective chart analysis.RESULTSForty clinical-grade EBV-CTL products from SCDs, related TPDs, or unrelated TPDs were generated for 37 patients with refractory EBV infections or EBV-associated malignancies with and without a history of transplantation, within 5 days (median) after donor identification. Thirty-four patients received 1-14 EBV-CTL products (fresh and cryopreserved). EBV-CTL transfer led to a complete response in 20 of 29 patients who were evaluated for clinical response. No infusion-related toxicity was reported. EBV-specific T cells in patients' blood were detectable in 16 of 18 monitored patients (89%) after transfer, and their presence correlated with clinical response.CONCLUSIONPersonalized clinical-grade manufacture of EBV-CTL products via immunomagnetic selection from SCDs, related TPDs, or unrelated TPDs in a timely manner is feasible. Overall, EBV-CTLs were clinically effective and well tolerated. Our data suggest EBV-CTL transfer as a promising therapeutic approach for immunocompromised patients with refractory EBV-associated diseases beyond HSCT, as well as patients with preexisting organ dysfunction.TRIAL REGISTRATIONNot applicable.FUNDINGThis study was funded in part by the German Research Foundation (DFG, 158989968/SFB 900), the Deutsche Kinderkrebsstiftung (DKS 2013.09), Wilhelm-Sander-Stiftung (reference 2015.097.1), Ellen-Schmidt-Program of Hannover Medical School, and German Federal Ministry of Education and Research (reference 01EO0802).
Keywords: Adaptive immunity; Immunotherapy; T cells; Therapeutics.
Figures


References
-
- Al Hamed R, et al. Epstein-Barr virus-related post-transplant lymphoproliferative disease (EBV-PTLD) in the setting of allogeneic stem cell transplantation: a comprehensive review from pathogenesis to forthcoming treatment modalities. Bone Marrow Transplant. 2020;55(1):25–39. doi: 10.1038/s41409-019-0548-7. - DOI - PubMed

