Pharmacological Characteristics of the Hydroethanolic Extract of Acmella oleracea (L) R. K. Jansen Flowers: ADME/Tox In Silico and In Vivo Antihypertensive and Chronic Toxicity Evaluation
- PMID: 37159592
- PMCID: PMC10163967
- DOI: 10.1155/2023/1278720
Pharmacological Characteristics of the Hydroethanolic Extract of Acmella oleracea (L) R. K. Jansen Flowers: ADME/Tox In Silico and In Vivo Antihypertensive and Chronic Toxicity Evaluation
Abstract
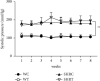
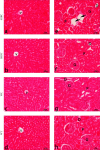
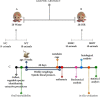
Acmella oleracea (L.) R. K. Jansen, popularly known as jambu in Northern Brazil, is widely used in folk medicine and local cuisine. Its consumption in different ways reinforces the need for safety assessments. In this study, the major compounds found in the hydroethanolic extract of A. oleracea flowers (EHFAO) were characterized by ultra-performance liquid mass spectrometry (UHPLC-ESI-QTOF-MS/MS). The effects of oral administration of 100/mg/kg of EHFAO extract over 60 days in male spontaneously hypertensive (SHR) and Wistar (WR) rats and the in silico ADME/Tox predictions, lipophilicity, and water solubility were accomplished for the compounds identified. Spilanthol was detected as the foremost major compound at a concentration of 97.7%, followed by 1.53% scopoletin and 0.77% d-limonene. The treatment with EHFAO did not alter the animals´ weight over the studied period. Moderate alterations were observed solely in the hepatic enzymes AST (WR = 97 UI/L and SHR = 150 UI/L ∗ p < 0.05) and ALT (WR = 55 UI/L and SHR = 95 UI/L ∗ p < 0.05), while no relevant histopathological alterations were found. The in-silico study confirmed the in vivo findings, as the identified compounds were considered highly bioactive orally, due to their drug similarity profiles, adequate lipid solubility, bioavailability, and pharmacokinetics. Therefore, the chronic treatment with EHFAO was found safe at the concentration of 100/mg/kg, with no interference in the blood pressure levels neither appreciable toxic effects.
Copyright © 2023 Emanuelle T. Rodrigues et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures







References
-
- Freitas Blanco V. S. D., Michalak B., Zelioli I. A. M., et al. Isolation of spilanthol from Acmella oleracea based on Green Chemistry and evaluation of its in vitro anti-inflammatory activity. The Journal of Supercritical Fluids . 2018;140:372–379. doi: 10.1016/j.supflu.2018.07.028. - DOI
-
- Balieiro O. C., da Silva Pinheiro M. S., Silva S. Y. S., et al. Analytical and preparative chromatographic approaches for extraction of spilanthol from Acmella oleracea flowers. Microchemical Journal . 2020;157 doi: 10.1016/j.microc.2020.105035.105035 - DOI
-
- de Souza G. C., Viana M. D., Goés L. D. M., et al. Reproductive toxicity of the hydroethanolic extract of the flowers of Acmella oleracea and spilanthol in zebrafish: in vivo and in silico evaluation. Human & Experimental Toxicology . 2020;39(2):127–146. doi: 10.1177/0960327119878257. - DOI - PubMed
LinkOut - more resources
Full Text Sources

