Generation of left ventricle-like cardiomyocytes with improved structural, functional, and metabolic maturity from human pluripotent stem cells
- PMID: 37159667
- PMCID: PMC10163040
- DOI: 10.1016/j.crmeth.2023.100456
Generation of left ventricle-like cardiomyocytes with improved structural, functional, and metabolic maturity from human pluripotent stem cells
Abstract
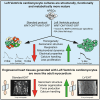
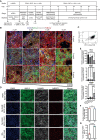
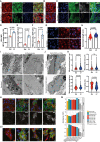
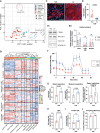
Decreased left ventricle (LV) function caused by genetic mutations or injury often leads to debilitating and fatal cardiovascular disease. LV cardiomyocytes are, therefore, a potentially valuable therapeutical target. Human pluripotent stem cell-derived cardiomyocytes (hPSC-CMs) are neither homogeneous nor functionally mature, which reduces their utility. Here, we exploit cardiac development knowledge to instruct differentiation of hPSCs specifically toward LV cardiomyocytes. Correct mesoderm patterning and retinoic acid pathway blocking are essential to generate near-homogenous LV-specific hPSC-CMs (hPSC-LV-CMs). These cells transit via first heart field progenitors and display typical ventricular action potentials. Importantly, hPSC-LV-CMs exhibit increased metabolism, reduced proliferation, and improved cytoarchitecture and functional maturity compared with age-matched cardiomyocytes generated using the standard WNT-ON/WNT-OFF protocol. Similarly, engineered heart tissues made from hPSC-LV-CMs are better organized, produce higher force, and beat more slowly but can be paced to physiological levels. Together, we show that functionally matured hPSC-LV-CMs can be obtained rapidly without exposure to current maturation regimes.
Keywords: cardiac progenitors; cardiomyocyte maturation; cardiomyocytes; differentiation; engineered heart tissues; human pluripotent stem cells; left ventricle; mesoderm; retinoic acid; ventricular.
Crown Copyright © 2023.
Conflict of interest statement
N.A.-G. is the vice president of research and development at AnaBios, San Diego. P.E.M. is a founding partner and the chief corporate development officer of AnaBios, San Diego. The Francis Crick Institute has filed a patent application related to this work (WO 2020/245612), and A.S.B. is listed as an inventor. The Francis Crick Institute has granted an exclusive license to Axol Bioscience to commercialize the protocol for the generation and sale of cardiomyocytes for R&D and the provision of contract research services. N.D., C.B., J.C.S., and A.S.B. may benefit from this license.
Figures


References
-
- Takasuna K., Asakura K., Araki S., Ando H., Kazusa K., Kitaguchi T., Kunimatsu T., Suzuki S., Miyamoto N. Comprehensive in vitro cardiac safety assessment using human stem cell technology: overview of CSAHi HEART initiative. J. Pharmacol. Toxicol. Methods. 2017;83:42–54. doi: 10.1016/j.vascn.2016.09.004. - DOI - PubMed
-
- Yang X., Papoian T. Moving beyond the comprehensive in vitro proarrhythmia assay: use of human-induced pluripotent stem cell-derived cardiomyocytes to assess contractile effects associated with drug-induced structural cardiotoxicity. J. Appl. Toxicol. 2018;38:1166–1176. doi: 10.1002/jat.3611. - DOI - PubMed
-
- Lian X., Zhang J., Azarin S.M., Zhu K., Hazeltine L.B., Bao X., Hsiao C., Kamp T.J., Palecek S.P. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/beta-catenin signaling under fully defined conditions. Nat. Protoc. 2013;8:162–175. doi: 10.1038/nprot.2012.150. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

