Safety and Immunogenicity of Radiation-Attenuated PfSPZ Vaccine in Equatoguinean Infants, Children, and Adults
- PMID: 37160281
- PMCID: PMC10324022
- DOI: 10.4269/ajtmh.22-0773
Safety and Immunogenicity of Radiation-Attenuated PfSPZ Vaccine in Equatoguinean Infants, Children, and Adults
Abstract
The radiation-attenuated Plasmodium falciparum sporozoites (PfSPZ) Vaccine has demonstrated safety and immunogenicity in 5-month-old to 50-year-old Africans in multiple trials. Except for one, each trial has restricted enrollment to either infants and children or adults < 50 years old. This trial was conducted in Equatorial Guinea and assessed the safety, tolerability, and immunogenicity of three direct venous inoculations of 1.8 × 106 or 2.7 × 106 PfSPZ, of PfSPZ Vaccine, or normal saline administered at 8-week intervals in a randomized, double-blind, placebo-controlled trial stratified by age (6-11 months and 1-5, 6-10, 11-17, 18-35, and 36-61 years). All doses were successfully administered. In all, 192/207 injections (93%) in those aged 6-61 years were rated as causing no or mild pain. There were no significant differences in solicited adverse events (AEs) between vaccinees and controls in any age group (P ≥ 0.17). There were no significant differences between vaccinees and controls with respect to the rates or severity of unsolicited AEs or laboratory abnormalities. Development of antibodies to P. falciparum circumsporozoite protein occurred in 67/69 vaccinees (97%) and 0/15 controls. Median antibody levels were highest in infants and 1-5-year-olds and declined progressively with age. Antibody responses in children were greater than in adults protected against controlled human malaria infection. Robust immunogenicity, combined with a benign AE profile, indicates children are an ideal target for immunization with PfSPZ Vaccine.
Trial registration: ClinicalTrials.gov NCT02859350.
Figures

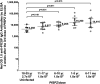
References
-
- World Health Organization , 2020. World Malaria Report 2020: 20 Years of Global Progress and Challenges. Geneva, Switzerland: WHO. Available at: https://www.who.int/teams/global-malaria-programme/reports/world-malaria.... Accessed April 20, 2023.
-
- World Health Organization , 2021. World Malaria Report 2021. Geneva, Switzerland: WHO. Available at: https://www.who.int/teams/global-malaria-programme/reports/world-malaria.... Accessed April 20, 2023.
-
- Seder RA. et al., 2013. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science 341: 1359–1365. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

