Reversion of breast epithelial polarity alterations caused by obesity
- PMID: 37160903
- PMCID: PMC10170133
- DOI: 10.1038/s41523-023-00539-w
Reversion of breast epithelial polarity alterations caused by obesity
Abstract
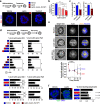
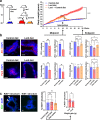
Molecular links between breast cancer risk factors and pro-oncogenic tissue alterations are poorly understood. The goal of this study was to characterize the impact of overweight and obesity on tissue markers of risk, using normal breast biopsies, a mouse model of diet-induced obesity, and cultured breast acini. Proliferation and alteration of epithelial polarity, both necessary for tumor initiation, were quantified by immunostaining. High BMI (>30) and elevated leptin were associated with compromised epithelial polarity whereas overweight was associated with a modest increase in proliferation in human and mice mammary glands. Human serum with unfavorable adipokine levels altered epithelial polarization of cultured acini, recapitulating the effect of leptin. Weight loss in mice led to metabolic improvements and restored epithelial polarity. In acini cultures, alteration of epithelial polarity was prevented by antioxidants and could be reverted by normalizing culture conditions. This study shows that obesity and/or dietary factors modulate tissue markers of risk. It provides a framework to set target values for metabolic improvements and to assess the efficacy of interventional studies aimed at reducing breast cancer risk.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
LinkOut - more resources
Full Text Sources

