Molecular mechanisms of tubulogenesis revealed in the sea star hydro-vascular organ
- PMID: 37160908
- PMCID: PMC10170166
- DOI: 10.1038/s41467-023-37947-2
Molecular mechanisms of tubulogenesis revealed in the sea star hydro-vascular organ
Abstract
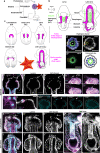
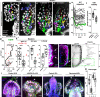
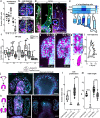
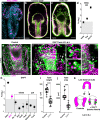
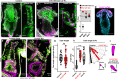
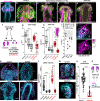
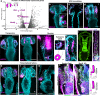
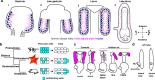
A fundamental goal in the organogenesis field is to understand how cells organize into tubular shapes. Toward this aim, we have established the hydro-vascular organ in the sea star Patiria miniata as a model for tubulogenesis. In this animal, bilateral tubes grow out from the tip of the developing gut, and precisely extend to specific sites in the larva. This growth involves cell migration coupled with mitosis in distinct zones. Cell proliferation requires FGF signaling, whereas the three-dimensional orientation of the organ depends on Wnt signaling. Specification and maintenance of tube cell fate requires Delta/Notch signaling. Moreover, we identify target genes of the FGF pathway that contribute to tube morphology, revealing molecular mechanisms for tube outgrowth. Finally, we report that FGF activates the Six1/2 transcription factor, which serves as an evolutionarily ancient regulator of branching morphogenesis. This study uncovers distinct mechanisms of tubulogenesis in vivo and we propose that cellular dynamics in the sea star hydro-vascular organ represents a key comparison for understanding the evolution of vertebrate organs.
© 2023. The Author(s).
Conflict of interest statement
Synthego provided free samples of FGFR gRNAs. Other gRNAs and all other products used in this study were purchased at market value. The authors declare no other competing interests.
Figures
References
Publication types
MeSH terms
Grants and funding
- K99 HD099315/HD/NICHD NIH HHS/United States
- R35 GM140897/GM/NIGMS NIH HHS/United States
- 1R35GM140897/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)
- K99HD099315/U.S. Department of Health & Human Services | NIH | Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

