Tunable [3+2] and [4+2] annulations for pyrrolidine and piperidine synthesis
- PMID: 37161704
- PMCID: PMC10297810
- DOI: 10.1039/d3cc01400b
Tunable [3+2] and [4+2] annulations for pyrrolidine and piperidine synthesis
Abstract
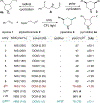
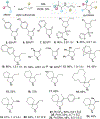
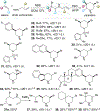
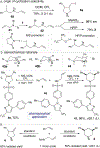
N-heterocycles are privileged pharmaceutical scaffolds in drug discovery and development. We disclose here divergent intermolecular coupling strategies that can access diverse N-heterocycles directly from olefins. The radical-to-polar mechanistic switching is key for the divergent cyclization processes. These distinctive annulations result in the coupling of alkenes with simple bifunctional reagents for divergent N-heterocycle syntheses.
Conflict of interest statement
Conflicts of interest
There are no conflicts to declare.
Figures

Similar articles
-
Bifunctional sulfilimines enable synthesis of multiple N-heterocycles from alkenes.Nat Chem. 2022 Aug;14(8):898-904. doi: 10.1038/s41557-022-00997-y. Epub 2022 Jul 25. Nat Chem. 2022. PMID: 35871706 Free PMC article.
-
Rhodium-Catalyzed C-H Alkenylation/Electrocyclization Cascade Provides Dihydropyridines That Serve as Versatile Intermediates to Diverse Nitrogen Heterocycles.Acc Chem Res. 2021 Apr 6;54(7):1766-1778. doi: 10.1021/acs.accounts.1c00027. Epub 2021 Mar 19. Acc Chem Res. 2021. PMID: 33740369 Free PMC article. Review.
-
Visible-Light-Catalyzed N-Radical-Enabled Cyclization of Alkenes for the Synthesis of Five-Membered N-Heterocycles.ChemSusChem. 2021 Nov 4;14(21):4658-4670. doi: 10.1002/cssc.202101586. Epub 2021 Sep 15. ChemSusChem. 2021. PMID: 34402206 Review.
-
Ni-Catalyzed Regioselective Dicarbofunctionalization of Unactivated Olefins by Tandem Cyclization/Cross-Coupling and Application to the Concise Synthesis of Lignan Natural Products.J Org Chem. 2018 Mar 2;83(5):2920-2936. doi: 10.1021/acs.joc.8b00184. Epub 2018 Feb 22. J Org Chem. 2018. PMID: 29446941
-
TBN-triggered, manipulable annulations of o-hydroxyarylenaminones for divergent syntheses of oximinochromanones and oximinocoumaranones.Chem Commun (Camb). 2021 Nov 19;57(92):12285-12288. doi: 10.1039/d1cc05389b. Chem Commun (Camb). 2021. PMID: 34730570
References
-
- Vitaku E, Smith DT, Njardarson JT, J. Med. Chem. 2014, 57, 10257–10274. - PubMed
-
-
For selected reviews on olefin difunctionalizations: Derosa J, Tran VT, van der Puyl VA, Engle KM, Aldrichimica. Acta. 2018, 51, 21–32.Wickham LM, Giri R, Acc. Chem. Res. 2021, 54, 3415–3437.Siu JC, Fu N, Lin S, Acc. Chem. Res. 2020, 53, 547–560.
-
-
-
For a selected review and early examples: Chemler SR, Fuller PH, Chem. Soc. Rev. 2007, 36, 1153–1160.Schlummer B, Hartwig JF, Org. Lett. 2002, 4, 1471–1474.Ney JE, Wolfe JP, Angew. Chem. Int. Ed. 2004, 43, 3605–3608.Michael FE, Cochran BM J. Am. Chem. Soc. 2006, 128, 4246–4248.Fuller PH, Kim J-W, Chemler SR, J. Am. Chem. Soc. 2008, 130, 17638–17639.Nguyen TM, Nicewicz DA, J. Am. Chem. Soc. 2013, 135, 9588–9591.
-
-
- Pandey G, Banerjee P, Gadre SR, Chem. Rev. 2006, 106, 4484–4517 - PubMed
-
- Yar M, McGarrigle EM, Aggarwal VK, Angew. Chem., Int. Ed. 2008, 47, 3784–3786. - PubMed
- Matlock JV, Svejstrup TD, Songara P, Overington S, McGarrigle EM, Aggarwal VK, Org. Lett. 2015, 17, 5044–5047. - PubMed
- Juliá F, Yan J, Paulus F, Ritter T, J. Am. Chem. Soc. 2021, 143, 12992–12998. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

