An Update on Adipose-Derived Stem Cells for Regenerative Medicine: Where Challenge Meets Opportunity
- PMID: 37162248
- PMCID: PMC10369252
- DOI: 10.1002/advs.202207334
An Update on Adipose-Derived Stem Cells for Regenerative Medicine: Where Challenge Meets Opportunity
Abstract
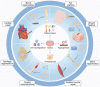
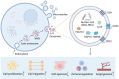
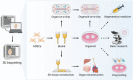
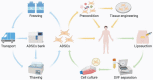
Over the last decade, adipose-derived stem cells (ADSCs) have attracted increasing attention in the field of regenerative medicine. ADSCs appear to be the most advantageous cell type for regenerative therapies owing to their easy accessibility, multipotency, and active paracrine activity. This review highlights current challenges in translating ADSC-based therapies into clinical settings and discusses novel strategies to overcome the limitations of ADSCs. To further establish ADSC-based therapies as an emerging platform for regenerative medicine, this review also provides an update on the advancements in this field, including fat grafting, wound healing, bone regeneration, skeletal muscle repair, tendon reconstruction, cartilage regeneration, cardiac repair, and nerve regeneration. ADSC-based therapies are expected to be more tissue-specific and increasingly important in regenerative medicine.
Keywords: 3D bioprinting; adipose-derived stem cells; cell-free therapy; clinical application; regenerative medicine.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Cossu G., Birchall M., Brown T., De Coppi P., Culme‐Seymour E., Gibbon S., Hitchcock J., Mason C., Montgomery J., Morris S., Muntoni F., Napier D., Owji N., Prasad A., Round J., Saprai P., Stilgoe J., Thrasher A., Wilson J., Lancet 2018, 391, 883. - PubMed
-
- Karagiannis P., Takahashi K., Saito M., Yoshida Y., Okita K., Watanabe A., Inoue H., Yamashita J. K., Todani M., Nakagawa M., Osawa M., Yashiro Y., Yamanaka S., Osafune K., Physiol. Rev. 2019, 9, 79. - PubMed
-
- Erices A., Conget P., Minguell J. J., Br. J. Haematol. 2000, 109, 235. - PubMed
-
- Igura K., Zhang X., Takahashi K., Mitsuru A., Yamaguchi S., Takashi T. A., Cytotherapy 2004, 6, 543. - PubMed
Publication types
MeSH terms
Grants and funding
- 82002321/National Natural Science Foundation of China
- 82072425/National Natural Science Foundation of China
- 82072498/National Natural Science Foundation of China
- 82272567/National Natural Science Foundation of China
- BK2021650/Natural Science Foundation of Jiangsu Province
- BE2020666/Natural Science Foundation of Jiangsu Province
- ZD2022021/Jiangsu Medical Research Project
- LCZX202003/Special Project of Diagnosis and Treatment Technology for Key Clinical Diseases in Suzhou
- KJXW2020005/Youth Program of Developing Public Health through Science and Education of Suzhou
- GSWS2022002/Gusu Health Talents Project in Suzhou
- the Priority Academic Program Development of Jiangsu Higher Education Institutions
- Jiangsu Provincial Medical Innovation Center
LinkOut - more resources
Full Text Sources
Medical
