Binary or Nonbinary Fission? Reproductive Mode of a Predatory Bacterium Depends on Prey Size
- PMID: 37162334
- PMCID: PMC10294633
- DOI: 10.1128/mbio.00772-23
Binary or Nonbinary Fission? Reproductive Mode of a Predatory Bacterium Depends on Prey Size
Abstract
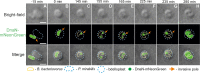
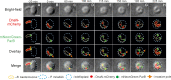
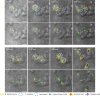
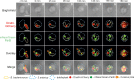
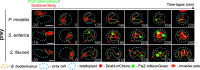
Most bacteria, including model organisms such as Escherichia coli, Bacillus subtilis, and Caulobacter crescentus, reproduce by binary fission. However, some bacteria belonging to various lineages, including antibiotic-producing Streptomyces and predatory Bdellovibrio, proliferate by nonbinary fission, wherein three or more chromosome copies are synthesized and the resulting multinucleoid filamentous cell subdivides into progeny cells. Here, we demonstrate for the first time that the predatory bacterium Bdellovibrio bacteriovorus reproduces through both binary and nonbinary fission inside different prey bacteria. Switching between the two modes correlates with the prey size. In relatively small prey cells, B. bacteriovorus undergoes binary fission; the FtsZ ring assembles in the midcell, and the mother cell splits into two daughter cells. In larger prey cells, B. bacteriovorus switches to nonbinary fission and creates multiple asynchronously assembled FtsZ rings to produce three or more daughter cells. Completion of bacterial cell cycle critically depends on precise spatiotemporal coordination of chromosome replication with other cell cycle events, including cell division. We show that B. bacteriovorus always initiates chromosome replication at the invasive pole of the cell, but the spatiotemporal choreography of subsequent steps depends on the fission mode and/or the number of progeny cells. In nonbinary dividing filaments producing five or more progeny cells, the last round(s) of replication may also be initiated at the noninvasive pole. Altogether, we find that B. bacteriovorus reproduces through bimodal fission and that extracellular factors, such as the prey size, can shape replication choreography, providing new insights about bacterial life cycles. IMPORTANCE Most eukaryotic and bacterial cells divide by binary fission, where one mother cell produces two progeny cells, or, rarely, by nonbinary fission. All bacteria studied to date use only one of these two reproduction modes. We demonstrate for the first time that a predatory bacterium, Bdellovibrio bacteriovorus, exhibits bimodal fission and the mode of division depends on the size of the prey bacterium inside which B. bacteriovorus grows. This work provides key insights into the mode and dynamics of B. bacteriovorus proliferation in different pathogens that pose a major threat to human health due to their emerging antibiotic resistance (Proteus mirabilis, Salmonella enterica, and Shigella flexneri). The use of predatory bacteria such as B. bacteriovorus is currently regarded as a promising strategy to kill antibiotic-resistant pathogens. We find that B. bacteriovorus employs different chromosome replication choreographies and division modes when preying on those pathogens. Our findings may facilitate the design of efficient pathogen elimination strategies.
Keywords: Bdellovibrio bacteriovorus; FtsZ; cell cycle; chromosome replication; predator.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Chromosome structure and DNA replication dynamics during the life cycle of the predatory bacterium Bdellovibrio bacteriovorus.FEMS Microbiol Rev. 2023 Nov 1;47(6):fuad057. doi: 10.1093/femsre/fuad057. FEMS Microbiol Rev. 2023. PMID: 37791401 Free PMC article. Review.
-
Dynamics of Chromosome Replication and Its Relationship to Predatory Attack Lifestyles in Bdellovibrio bacteriovorus.Appl Environ Microbiol. 2019 Jul 1;85(14):e00730-19. doi: 10.1128/AEM.00730-19. Print 2019 Jul 15. Appl Environ Microbiol. 2019. PMID: 31076424 Free PMC article.
-
Distinct dynamics and proximity networks of hub proteins at the prey-invading cell pole in a predatory bacterium.J Bacteriol. 2024 Apr 18;206(4):e0001424. doi: 10.1128/jb.00014-24. Epub 2024 Mar 12. J Bacteriol. 2024. PMID: 38470120 Free PMC article.
-
An MltA-Like Lytic Transglycosylase Secreted by Bdellovibrio bacteriovorus Cleaves the Prey Septum during Predatory Invasion.J Bacteriol. 2023 Apr 25;205(4):e0047522. doi: 10.1128/jb.00475-22. Epub 2023 Apr 3. J Bacteriol. 2023. PMID: 37010281 Free PMC article.
-
Advances in cellular and molecular predatory biology of Bdellovibrio bacteriovorus six decades after discovery.Front Microbiol. 2023 May 15;14:1168709. doi: 10.3389/fmicb.2023.1168709. eCollection 2023. Front Microbiol. 2023. PMID: 37256055 Free PMC article. Review.
Cited by
-
Chromosome structure and DNA replication dynamics during the life cycle of the predatory bacterium Bdellovibrio bacteriovorus.FEMS Microbiol Rev. 2023 Nov 1;47(6):fuad057. doi: 10.1093/femsre/fuad057. FEMS Microbiol Rev. 2023. PMID: 37791401 Free PMC article. Review.
-
Lifecycle of a predatory bacterium vampirizing its prey through the cell envelope and S-layer.Nat Commun. 2024 Apr 27;15(1):3590. doi: 10.1038/s41467-024-48042-5. Nat Commun. 2024. PMID: 38678033 Free PMC article.
-
Cultivation of filamentous fungi in airlift bioreactors: advantages and disadvantages.Appl Microbiol Biotechnol. 2025 Feb 10;109(1):41. doi: 10.1007/s00253-025-13422-4. Appl Microbiol Biotechnol. 2025. PMID: 39928147 Free PMC article. Review.
-
Allocation of resources among multiple daughter cells.bioRxiv [Preprint]. 2025 May 3:2025.05.02.651883. doi: 10.1101/2025.05.02.651883. bioRxiv. 2025. PMID: 40654981 Free PMC article. Preprint.
References
-
- Fenton AK, Kanna M, Woods RD, Aizawa S-I, Sockett RE. 2010. Shadowing the actions of a predator: backlit fluorescent microscopy reveals synchronous nonbinary septation of predatory Bdellovibrio inside prey and exit through discrete bdelloplast pores. J Bacteriol 192:6329–6335. doi:10.1128/JB.00914-10. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

